Abstract
Purpose
To evaluate the efficacy of intravitreal dexamethasone implants for the treatment of macular edema (ME) due to branch retinal vein occlusion (BRVO) according to the duration of symptoms.
Methods
Thirty-one patients who received an intravitreal dexamethasone implant for ME due to BRVO were included in this ret-rospective study. The patients were divided into 2 groups. Group A included eyes with symptom duration less than 12 weeks and Group B included eyes with symptom duration of 12 weeks or longer. The main efficacy outcomes such as best-corrected visual acui-ty (BCVA) and central macular thickness (CMT) were measured at baseline and every 4 weeks over 24 weeks. Retreatment criteria included an increased CMT of 150 µm or reduction of logarithm of the minimal angle of resolution (log MAR) scores of at least 0.2.
Results
The CMT and BCVA improved significantly at each follow-up compared with the baseline ( p < 0.001, p < 0.05, re-spectively). At 4 and 8 weeks after the first injection, CMT in Group A (285.3 ± 19.9, 276.1 ± 23.1 μ m, respectively) was less than in Group B (310.3 ± 37.5, 318.1 ± 39.6 μ m; p = 0.033, p = 0.001, respectively). At 4 weeks, the BCVA in Group A was better than in Group B ( p = 0.009). Rate and timing of recurrence were not different between the 2 groups ( p > 0.05). At 24 weeks, Group A showed less CMT and better BCVA compared with Group B ( p = 0.043, p = 0.041, respectively).
Conclusions
The intravitreal dexamethasone implant significantly reduced the CMT and improved BCVA in patients with ME due to BRVO. Patients with shorter symptom duration showed better anatomical and functional outcomes over 24 weeks. Early treatment with the intravitreal dexamethasone implant could produce better clinical results.
References
1. Campochiaro PA, Heier JS, Feiner L. . Ranibizumab for mac-ular edema following branch retinal vein occlusion: six-month pri-mary end point results of a phase III study. Ophthalmology. 2010; 117:1102–12.e1.
2. Scott IU, Ip MS, VanVeldhuisen PC. . A randomized trial com-paring the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009; 127:1115–28.
3. Argon laser photocoagulation for macular edema in branch vein occlusion The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984; 98:271–82.
4. CATT Research Group, Martin DF, Maguire MG. . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–908.

5. Kuppermann BD, Blumenkranz MS, Haller JA. . Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007; 125:309–17.

6. Haller JA, Bandello F, Belfort R Jr. . Dexamethasone intra-vitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–60.
7. Coscas G, Coscas F, Zucchiatti I. . SD-OCT pattern of retinal venous occlusion with cystoid macular edema treated with Ozurdex(R). Eur J Ophthalmol. 2011; 21:631–6.
8. Haller JA, Bandello F, Belfort R Jr. . Randomized, sham-con-trolled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.

9. Bezatis A, Spital G, Höhn F. . Functional and anatomical re-sults after a single intravitreal Ozurdex injection in retinal vein oc-clusion: a 6-month follow-up-the SOLO study. Acta Ophthalmol. 2013; 91–e340-7.
10. Joshi L, Yaganti S, Gemenetzi M. . Dexamethasone implants in retinal vein occlusion: 12-month clinical effectiveness using repeat injections as-needed. Br J Ophthalmol. 2013; 97:1040–4.

11. Matonti F, Meyer F, Guigou S. . Ozurdex in the management of the macular edema following retinal vein occlusion in clinical practice. Acta Ophthalmol. 2013; 91:e584–6.

12. Patz A. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984; 98:374–5.

13. Kim YG, Kim ES, Kim MS. . Early and late intravitreal bev-acizumab injection in macular edema due to branch retinal vein occlusion. J Korean Ophthalmol Soc. 2009; 50:1527–30.

14. Oh JY, Seo JH, Ahn JK. . Early versus late intravitreal tri-amcinolone acetonide for macular edema associated with branch retinal vein occlusion. Korean J Ophthalmol. 2007; 21:18–20.

15. Kondo M, Kondo N, Ito Y. . Intravitreal injection of bev-acizumab for macular edema secondary to branch retinal vein oc-clusion: results after 12 months and multiple regression analysis. Retina. 2009; 29:1242–8.
16. Hikichi T, Higuchi M, Matsushita T. . Two-year outcomes of intravitreal bevacizumab therapy for macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2014; 98:195–9.

17. Demir M, Oba E, Gulkilik G. . Intravitreal bevacizumab for macular edema due to branch retinal vein occlusion: 12-month results. Clin Ophthalmol. 2011; 5:745–9.

18. Karagiannis DA, Karampelas MD, Soumplis VM. . Recurrence of macular edema in retinal vein occlusions after treatment with in-travitreal ranibizumab (Lucentis). Can J Ophthalmol. 2011; 46:486–90.

19. Mayer WJ, Wolf A, Kernt M. . Twelve-month experience with Ozurdex for the treatment of macular edema associated with retinal vein occlusion. Eye (Lond). 2013; 27:816–22.

20. Stefansson E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001; 79:435–40.
21. Larsson J, Bauer B, Andréasson S. The 30-Hz flicker cone ERG for monitoring the early course of central retinal vein occlusion. Acta Ophthalmol Scand. 2000; 78:187–90.

22. Kalogeropoulos C, Donati G, Pizzolato GP, Pournaras CJ. Morphology of early retinal lesions after experimental venous occlusion. Klin Monbl Augenheilkd. 1996; 208:375–6.
Figure 1.
Central macular thickness (CMT) changes after in-travitreal dexamethasone implant. CMT changes was sig-nificantly different from the baseline at all follow-up visits. * p < 0.001.
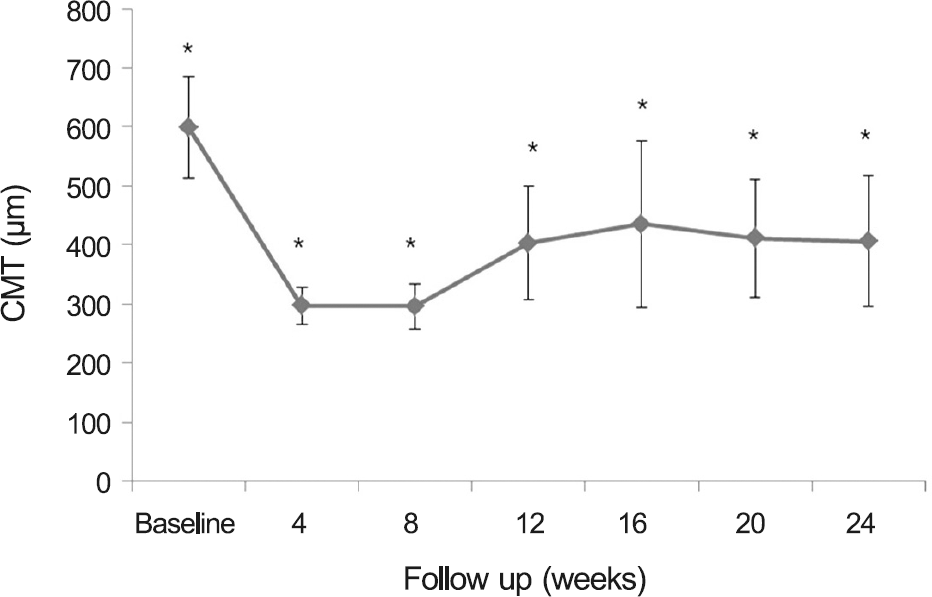
Figure 2.
(A) Comparison of central macular thickness (CMT) and (B) best-corrected visual acuity (BCVA) in patients with macular edema due to retinal vein occlusion after first in-jection of intravitreal dexamethasone according to symptom duration. (A) There was a significant difference at 4 ( p = 0.033), 8 ( p = 0.001) and 24 ( p = 0.043) weeks in CMT between two groups. (B) There was a significant difference at 4 ( p = 0.009) and 24 ( p = 0.041) weeks in the BCVA. Vertical bars are standard deviation of the Group A (symptom duration <12 weeks) and Group B (symptom duration ≥12 weeks). * p < 0.05.
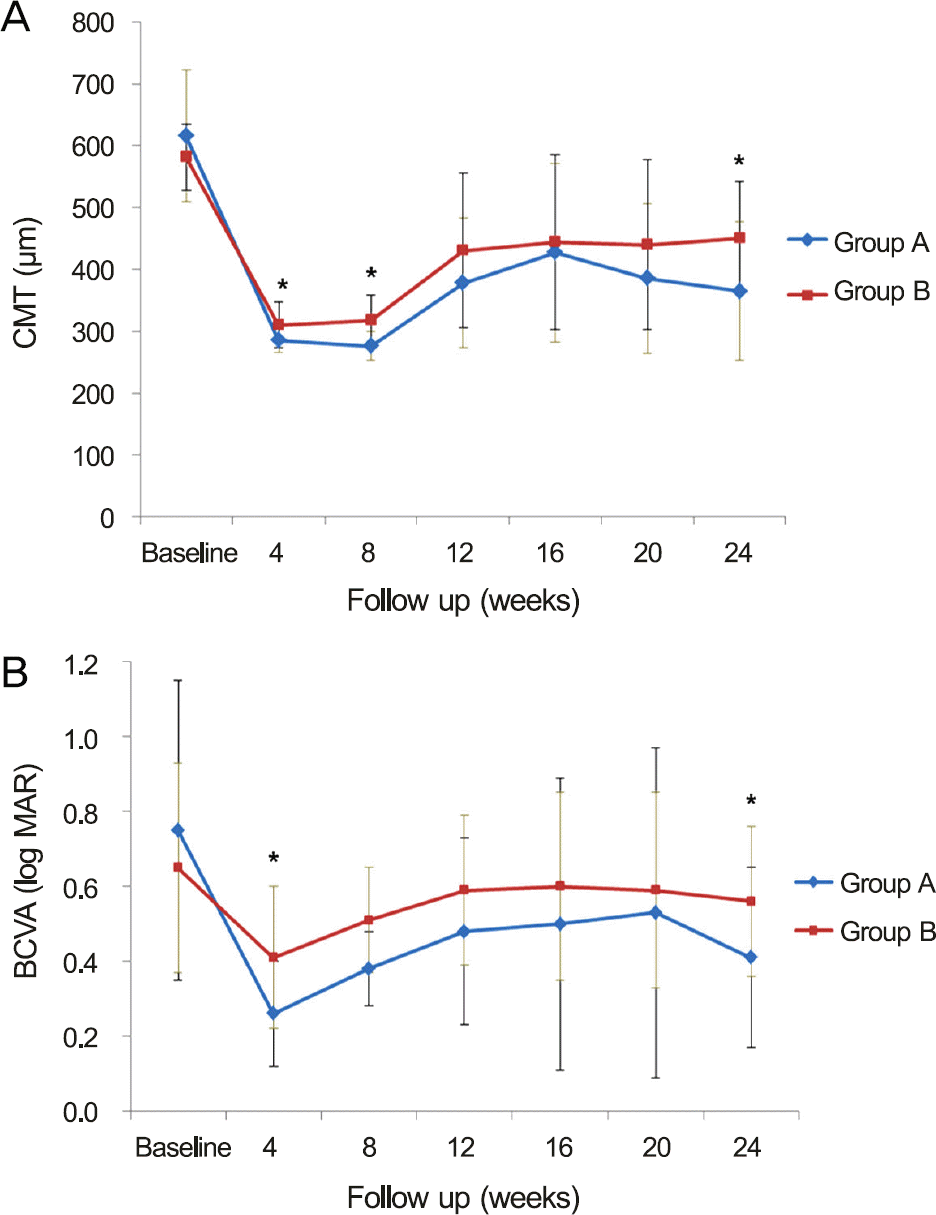
Table 1.
Clinical characteristics of the patients
| Group A∗ (16 eyes) | Group B†(15 eyes) | p-value | |
|---|---|---|---|
| Age (years) | 59.7 ± 8.9 | 65.3 ± 8.1 | 0.08‡ |
| Male gender (n, %) | 30 (55.5) | 25 (49.0) | 0.20§ |
| Symptom duration (weeks) | 7.7 ± 3.0 | 32.1 ± 19.4 | <0.001‡ |
| Mean follow up (weeks) | 25.7 ± 1.7 | 25.1 ± 1.1 | 0.32‡ |
| Hypertension (n, %) | 6 (37.5) | 5 (33.3) | 0.81§ |
Table 2.
Changes of central macular thickness and best-corrected visual acuity of total patients
Table 3.
Comparison of clinical outcomes according to symptom duration
| Group A∗ (16 eyes) | Group B† (16 eyes) | p-value | |
|---|---|---|---|
| Recurrence rate (%) | 56.2 | 60.0 | 0.80‡ |
| Timing of recurrence (weeks) | 14.7 ± 2.8 | 13.3 ± 2.0 | 0.34§ |
| Symptom duration (weeks) | 7.7 ± 3.0 | 32.1 ± 19.4 | <0.001§ |
| Final CMT (μ m) | 364.8 ± 112.5 | 450.9 ± 90.6 | 0.043§ |
| Final BCVA (log MAR) | 0.49 ± 0.25 | 0.61 ± 0.17 | 0.041§ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download