Abstract
Purpose
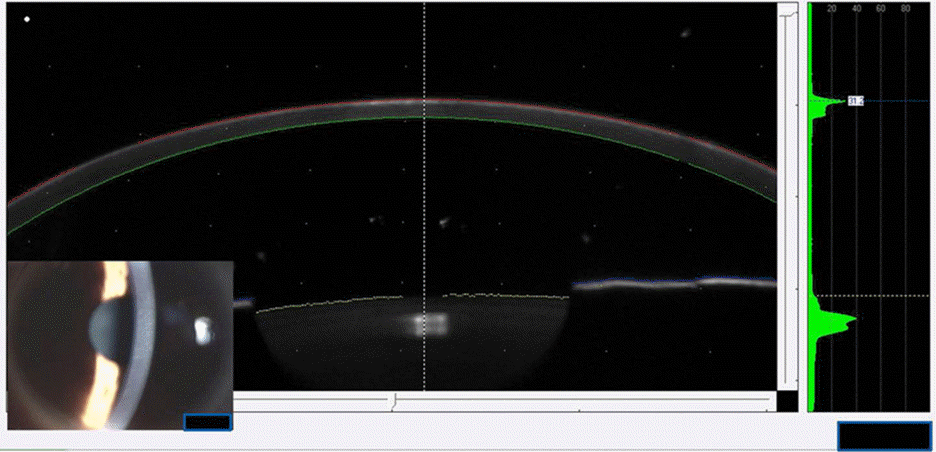
To evaluate the inhibitory effect of tranilast on formation of corneal haze using the Pentacam® after photorefractive keratectomy (PRK).
Methods
A prospective, randomized, paired eye study was performed. A total of 60 eyes from 30 patients were enrolled in the present study. Eyes were categorized as myopic eyes <-5 D and ≥-5 D. Patients undergoing PRK were randomized to receive tranilast in one eye and no medication in the contralateral eye. Three months postoperatively, corneal haze was measured with the Pentacam® and compared between the 2 groups.
Results
Statistical differences were not found in preoperative data in the tranilast or control groups (all p > 0.05). There was a strong decreasing density trend from the apex to the 3 mm radius in both groups (p < 0.05). However, postoperative corneal haze in the tranilast group was similar to the control group (p > 0.05).
References
1. Caubet E. Course of subepithelial corneal haze over 18 months after photorefractive keratectomy for myopia [corrected]. Refract Corneal Surg. 1993; 9:S65–70.

2. Gartry DS, Muir MG, Lohmann CP, Marshall J. The effect of topical corticosteroids on refractive outcome and corneal haze after photorefractive keratectomy. A prospective, randomized, double-blind trial. Arch Ophthalmol. 1992; 110:944–52.
3. Seiler T, Derse M, Pham T. Repeated excimer laser treatment after photorefractive keratectomy. Arch Ophthalmol. 1992; 110:1230–3.

4. Song JS, Jung HR, Kim HM. Effects of topical tranilast on corneal haze after photorefractive keratectomy. J Cataract Refract Surg. 2005; 31:1065–73.

5. Wilson SE, Walker JW, Chwang EL, He YG. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993; 34:2544–61.
6. Grant MB, Khaw PT, Schultz GS, et al. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci. 1992; 33:3292–301.
7. Ohji M, SundarRaj N, Thoft RA. Transforming growth factor-beta stimulates collagen and fibronectin synthesis by human corneal stromal fibroblasts in vitro. Curr Eye Res. 1993; 12:703–9.
8. Fini ME, Girard MT, Matsubara M, Bartlett JD. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest Ophthalmol Vis Sci. 1995; 36:622–33.
9. Netto MV, Mohan RR, Ambrósio R Jr, et al. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005; 24:509–22.
10. Chen C, Michelini-Norris B, Stevens S, et al. Measurement of mRNAs for TGFss and extracellular matrix proteins in corneas of rats after PRK. Invest Ophthalmol Vis Sci. 2000; 41:4108–16.
11. Myers JS, Gomes JA, Siepser SB, et al. Effect of transforming growth factor beta 1 on stromal haze following excimer laser photorefractive keratectomy in rabbits. J Refract Surg. 1997; 13:356–61.
12. Platten M, Ho PP, Youssef S, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005; 310:850–5.

13. Tani E, Katakami C, Negi A. Effects of various eye drops on corneal wound healing after superficial keratectomy in rabbits. Jpn J Ophthalmol. 2002; 46:488–95.

14. Furukawa H, Nakayasu K, Gotoh T, et al. Effect of topical tranilast and corticosteroids on subepithelial haze after photorefractive keratectomy in rabbits. J Refract Surg. 1997; 13:S457–8.

15. Lee JE, Han HJ, Lee JS, Oum BS. Effect of tranilast on the proliferation of human corneal keratocytes in vitro. J Korean Ophthalmol Soc. 2005; 46:510–20.
16. Fantes FE, Hanna KD, Waring GO 3rd, et al. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990; 108:665–75.

17. van de Pol C, Soya K, Hwang DG. Objective assessment of transient corneal haze and its relation to visual performance after photorefractive keratectomy. Am J Ophthalmol. 2001; 132:204–10.

18. Takacs AI, Mihaltz K, Nagy ZZ. Corneal density with the Pentacam after photorefractive keratectomy. J Refract Surg. 2011; 27:269–77.

19. Mirza MA, Qazi MA, Pepose JS. Treatment of dense subepithelial corneal haze after laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2004; 30:709–14.

20. Nagy ZZ, Tóth J, Nagymihály A, Süveges I. The role of ultraviolet-B in corneal healing following excimer laser in situ keratomileusis. Pathol Oncol Res. 2002; 8:41–6.

21. Nagy ZZ, Hiscott P, Seitz B, et al. Clinical and morphological response to UV-B irradiation after excimer laser photorefractive keratectomy. Surv Ophthalmol. 1997; 42:S64–76.

22. Jeong BJ, Kim HH, Park YJ, et al. Effect of mitomycin C to inhibit corneal haze formation after photorefractive keratectomy for high myopia. J Korean Ophthalmol Soc. 2006; 47:725–34.
23. Kim ES, Jin KH. Evaluation of the prophylactic use of mitomycin to inhibit haze formation after LASEK. J Korean Ophthalmol Soc. 2007; 48:623–9.
24. Mita T, Yamashita H, Kaji Y, et al. Effects of transforming growth factor beta on corneal epithelial and stromal cell function in a rat wound healing model after excimer laser keratectomy. Graefes Arch Clin Exp Ophthalmol. 1998; 236:834–43.
25. Lee JH, Kim ET, Oh JH. The inhibitory effect of TGF-β inhibitor on the corneal opacity after corneal laceration. J Korean Ophthalmol Soc. 2009; 50:450–61.

26. Vetrugno M, Maino A, Quaranta GM, Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. Acta Ophthalmol Scand. 2001; 79:23–7.

27. Kim TI, Tchah H, Lee SA, et al. Apoptosis in keratocytes caused by mitomycin C. Invest Ophthalmol Vis Sci. 2003; 44:1912–7.

28. Kim TI, Pak JH, Lee SY, Tchah H. Mitomycin C-induced reduction of keratocytes and fibroblasts after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2004; 45:2978–84.

29. Kim HY, Lee HI, Chun YS, Kim JC. The effectiveness of tranilast in the prevention of posterior capsular opacity. J Korean Ophthalmol Soc. 2008; 49:1981–8.

30. Waseda T. [Concentrations of tranilast in keloid tissues]. Nihon Hifuka Gakkai Zasshi. 1989; 99:1159–65.
31. Suzawa H, Kikuchi S, Ichikawa K, Koda A. Inhibitory action of tranilast, an anti-allergic drug, on the release of cytokines and PGE2 from human monocytes-macrophages. Jpn J Pharmacol. 1992; 60:85–90.
32. Suzawa H, Kikuchi S, Arai N, Koda A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. Jpn J Pharmacol. 1992; 60:91–6.
33. Isaji M, Aruga N, Naito J, Miyata H. Inhibition by tranilast of collagen accumulation in hypersensitive granulomatous inflammation in vivo and of morphological changes and functions of fibroblasts in vitro. Life Sci. 1994; 55:PL287–92.
34. Okamoto S, Sakai T, Iwaki Y, et al. Effects of tranilast on cultured rabbit corneal keratocytes and corneal haze after photorefractive keratectomy. Jpn J Ophthalmol. 1999; 43:355–62.

35. Nagy ZZ, Németh J, Süveges I, Csákány B. [Examination of subepithelial scar formation after photorefractive keratectomy with the ultrasound biomicroscope]. Klin Monbl Augenheilkd. 1996; 209:283–5.
36. Pei X, Bao Y, Chen Y, Li X. Correlation of lens density measured using the Pentacam Scheimpflug system with the Lens Opacities Classification System III grading score and visual acuity in age-related nuclear cataract. Br J Ophthalmol. 2008; 92:1471–5.

37. Grewal D, Jain R, Brar GS, Grewal SP. Pentacam tomograms: a novel method for quantification of posterior capsule opacification. Invest Ophthalmol Vis Sci. 2008; 49:2004–8.

38. Matsuda J, Hieda O, Kinoshita S. [Quantification of corneal opacity after refractive corneal surgery using the anterior segment analyzer]. Nihon Ganka Gakkai Zasshi. 2007; 111:447–53.
Figure 2.
Correlation of corneal haze with distance from the apex for myopic eyes <- 5 D after photorefractive keratectomy (PRK) in tranilast (p < 0.05, r = −0.64).
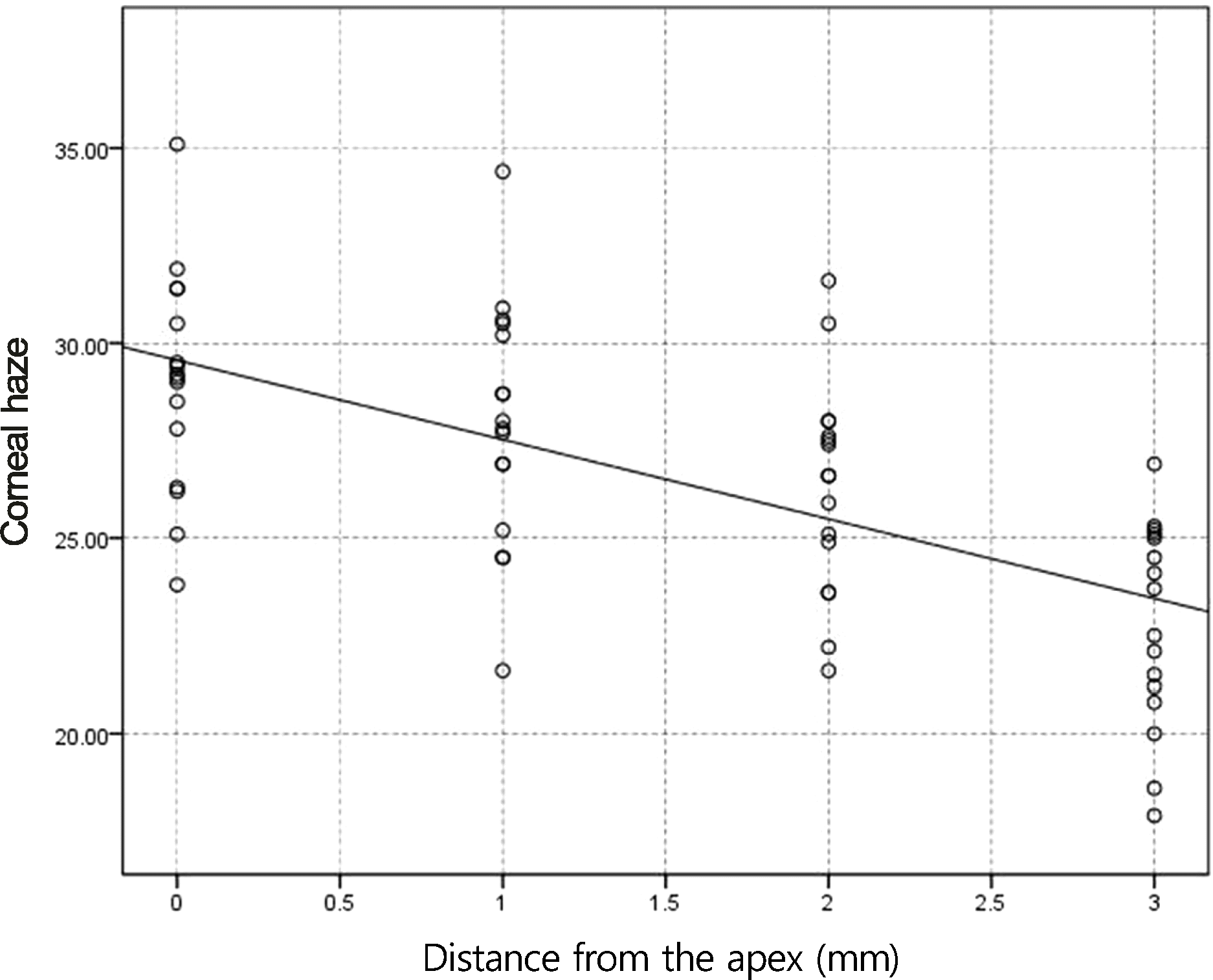
Figure 3.
Correlation of corneal haze with distance from the apex for myopic eyes <- 5 D after photorefractive keratectomy (PRK) in control (p < 0.05, r = −0.66).
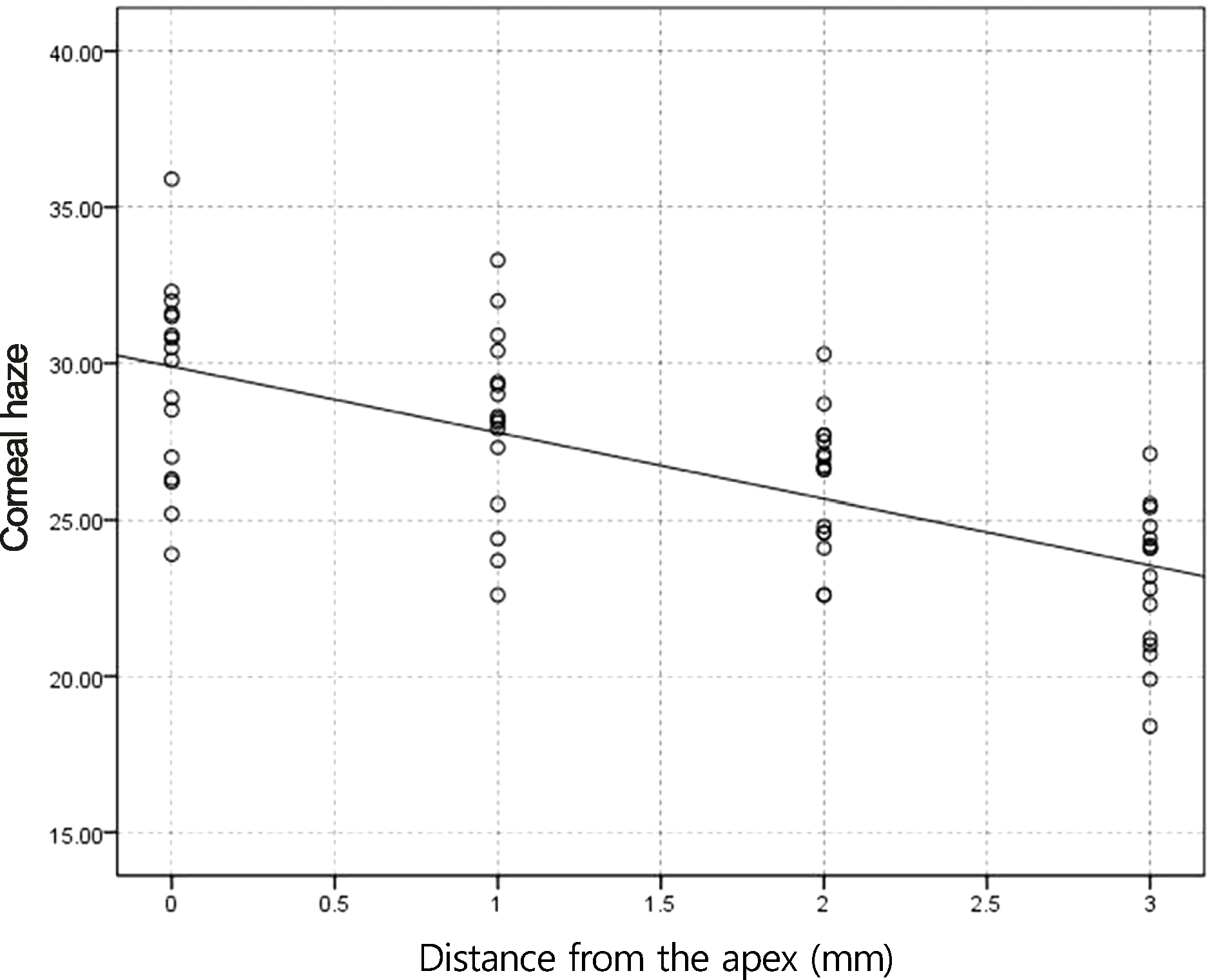
Figure 4.
Correlation of corneal haze with distance from the apex for myopic eyes ≥-5 D after photorefractive keratectomy (PRK) in tranilast (p < 0.05, r = −0.72).
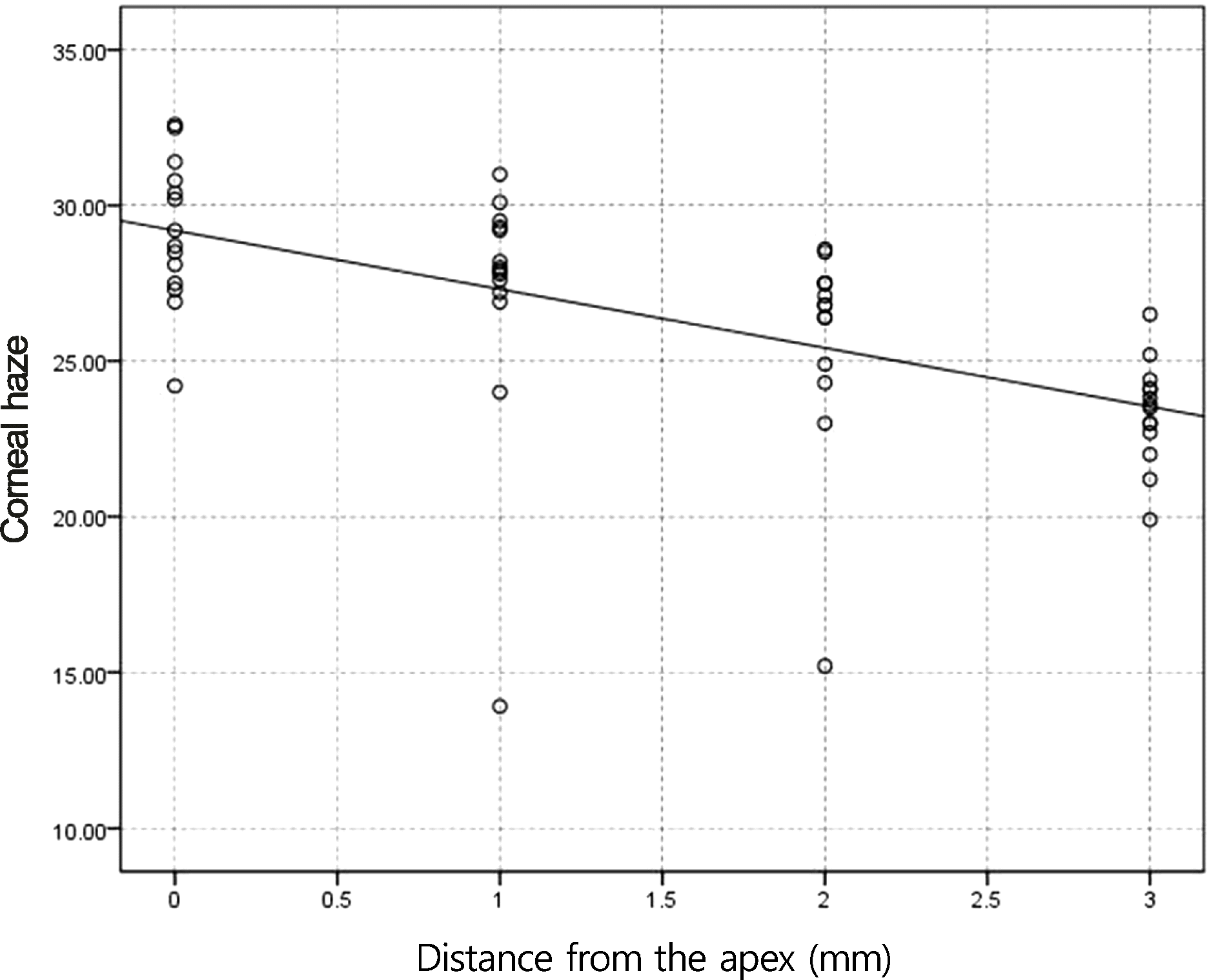
Figure 5.
Correlation of corneal haze with distance from the apex for myopic eyes ≥-5 D after photorefractive keratectomy (PRK) in control (p < 0.05, r = −0.69).
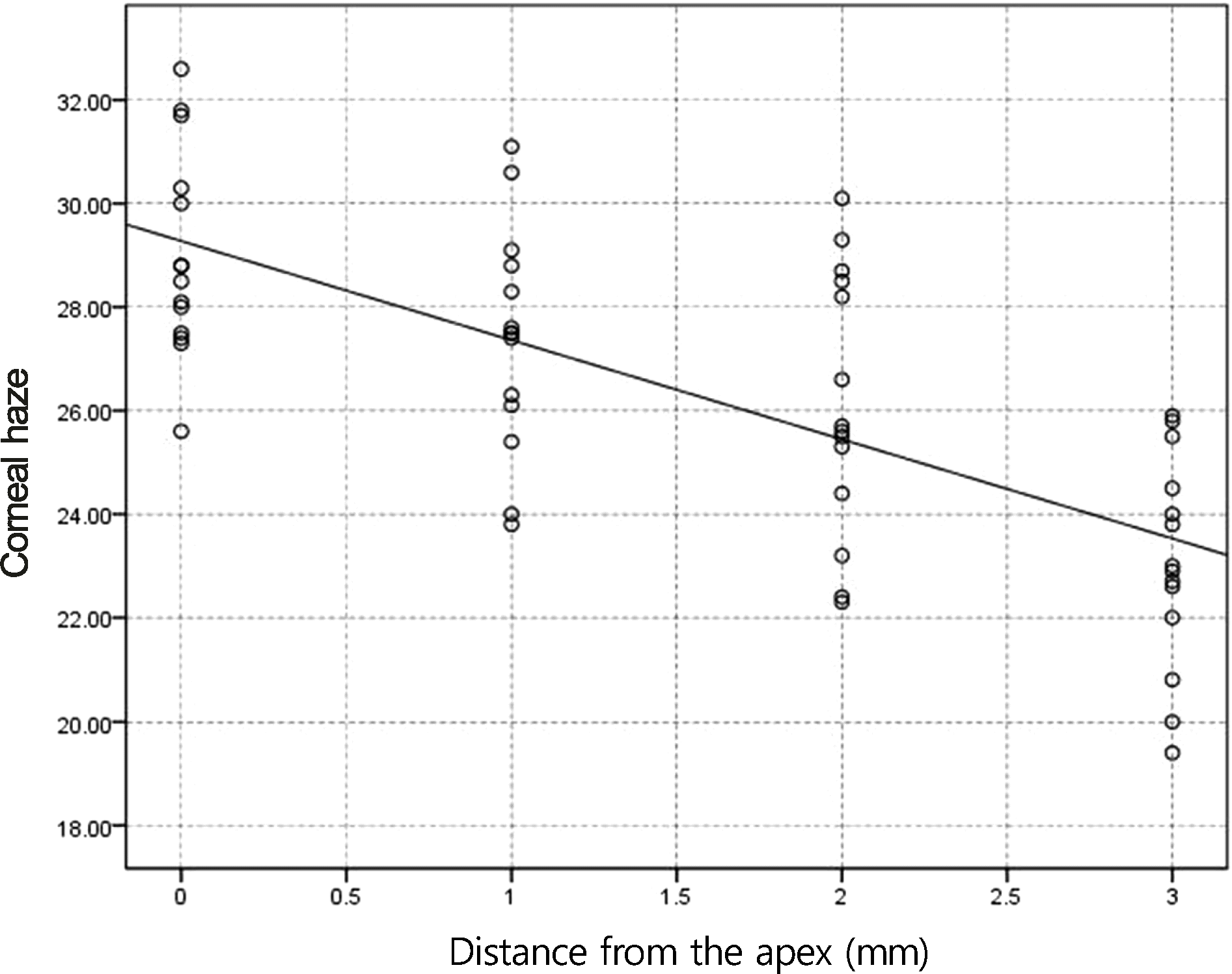
Table 1.
Preoperative data of myopic eyes <-5 D
Table 2.
Postoperative data of myopic eyes <-5 D
Table 3.
Mean maximum density values measured with the Pentacam® for myopic eyes <-5 D
| Distance from apex | Tranilast | Control | p-value |
|---|---|---|---|
| 0 mm | 29.01 ± 2.82 | 29.50 ± 3.13 | 0.46 |
| 1 mm | 27.94 ± 3.10 | 28.14 ± 2.94 | 0.69 |
| 2 mm | 26.29 ± 2.75 | 26.20 ± 2.14 | 0.83 |
| 3 mm | 22.78 ± 2.62 | 23.07 ± 2.33 | 0.39 |
Table 4.
Preoperative data of myopic eyes ≥-5 D
Table 5.
Postoperative data of myopic eyes ≥-5 D




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download