Abstract
Purpose
To investigate the shifting trends of pathogenic organisms, antibiotic resistance, and clinical characteristics of patients with Gram-positive bacterial keratitis and to elucidate the prognostic factors.
Methods
We performed a retrospective chart review of 152 isolates in 146 eyes with Gram-positive bacterial keratitis between January 1998 and December 2012. The study was divided into 5 periods for analysis of the bacteriological profiles and in vitro antibiotic resistance. The epidemiological and clinical characteristics were compared according to bacterial isolates. Logistic regression analysis was performed to determine the risk factors.
Results
Gram-positive bacterial keratitis tended to decrease and significant change in the distribution of isolates was not observed. Commonly isolated organisms were S. epidermidis (48.7%), S. aureus (25.0%), and S. pneumoniae (7.2%) in order of frequency. The resistance to fluoroquinolone tended to increase (p = 0.104) and resistance to gentamicin was significantly decreased (p = 0.01). S. epidermidis had the shortest corneal epithelium healing time (p = 0.035) and the most favorable visual outcome after treatment (p = 0.035) compared with the other species. Risk factors for poor visual outcomes included a best corrected visual acuity less than 0.1 at initial evaluation and an epithelial healing time greater than 10 days.
Conclusions
Gram-positive bacterial keratitis tended to decrease and S. epidermidis was the most common isolate. The clinical prognosis was most favorable in S. epidermidis. The BCVA less than 0.1 at initial evaluation was an important risk factor for poor visual outcome and surgical treatment in Gram-positive bacterial keratitis.
References
1. Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology. 2012; 19:1785–90.
3. Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001; 85:842–7.

4. Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980; 90:38–47.

5. Sun HJ, Lee JY, Kim SY, Jung MS. Clinical features of infectious keratitis in west coast area of Chungcheongnam-do, Korea. J Korean Ophthalmol Soc. 2010; 51:658–63.

6. Lim SH, Lee SB. Analysis of inpatients with bacterial keratitis over a 12-year period: pathogenic organisms and antibiotic resistance. J Korean Ophthalmol Soc. 2012; 53:372–84.

7. Hahn YH, Lee SJ, Hahn TW, et al. Antibiotic susceptibilities of ocular isolates from patients with bacterial keratitis: a multi-center study. J Korean Ophthalmol Soc. 1999; 40:2401–10.
8. Kim WJ, Kweon EY, Lee DW, et al. Prognostic factor and antibiotic susceptibility in bacterial keratitis: results of an eight-year period. J Korean Ophthalmol Soc. 2009; 50:1495–504.

9. Fong CF, Hu FR, Tseng CH, et al. Antibiotic susceptibility of bacterial isolates from bacterial keratitis cases in a university hospital in Taiwan. Am J Ophthalmol. 2007; 144:682–9.

10. Shalchi Z, Gurbaxani A, Baker M, Nash J. Antibiotic resistance in microbial keratitis: ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology. 2011; 118:2161–5.

11. Toshida H, Kogure N, Inoue N, Murakami A. Trends in microbial keratitis in Japan. Eye Contact Lens. 2007; 33:70–3.

12. Zhang C, Liang Y, Deng S, et al. Distribution of bacterial keratitis and emerging resistance to antibiotics in China from 2001 to 2004. Clin Ophthalmol. 2008; 2:575–9.
13. Yeh DL, Stinnett SS, Afshari NA. Analysis of bacterial cultures in infectious keratitis, 1997 to 2004. Am J Ophthalmol. 2006; 142:1066–8.

14. Mantadakis E, Maraki S, Michailidis L, et al. Antimicrobial susceptibility of Gram-positive cocci isolated from patients with conjunctivitis and keratitis in Crete, Greece. J Microbiol Immunol Infect. 2013; 46:41–7.

15. Green MD, Apel AJ, Naduvilath T, Stapleton FJ. Clinical outcomes of keratitis. Clin Experiment Ophthalmol. 2007; 35:421–6.

16. Park JH, Lee SB. Analysis on inpatients with infectious keratitis: causative organisms, clinical aspects and risk factors. J Korean Ophthalmol Soc. 2009; 50:1152–66.

17. Kim SJ, Lee SB. Analysis on elderly inpatients with infectious keratitis: causative organisms, clinical aspects, and risk factors. J Korean Ophthalmol Soc. 2010; 51:1554–67.

18. Kim JY, Yoon KC, Park YG, et al. Age-related clinical analysis of infectious keratitis in two tertiary centers. J Korean Ophthalmol Soc. 2010; 51:927–34.

19. Yoon JH, Jung JW, Moon HS, et al. Antibiotics susceptibility in bacterial keratitis and proper initial treatment. J Korean Ophthalmol Soc. 2013; 54:38–45.

20. Mukerji N, Vajpayee RB, Sharma N. Technique of area measurement of epithelial defects. Cornea. 2003; 22:549–51.

21. Biemer JJ. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann Clin Lab Sci. 1973; 3:135–40.
22. Jorgensen JH, Hindler JF. New consensus guidelines from the Clinical and Laboratory Standards Institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin Infect Dis. 2007; 44:280–6.

23. Shin SY, Koo SH, Kwon KC, et al. Evaluation of the Vitek 2 Korean antimicrobial susceptibility testing cards AST N056 and AST N055. Korean J Clin Microbiol. 2008; 11:23–8.

24. Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003; 87:834–8.

25. Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009; 57:273–9.

26. Jeong JG, Kweon EY, Cho NC, You IC. Comparison of methicillin-sensitive Staphylococcus epidermidis (MSSE) keratits and methicillin-resistant Staphylococcus epidermidis (MRSE) keratitis. J Korean Ophthalmol Soc. 2011; 52:930–5.
27. Sotozono C, Inagaki K, Fujita A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002; 21(7 Suppl):S94–101.

28. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005; 352:1436–44.
29. Adebayo A, Parikh JG, McCormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial conjunctival isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol. 2011; 249:111–9.

30. Chalita MR, Höfling-Lima AL, Paranhos A Jr, et al. Shifting trends in in vitro antibiotic susceptibilities for common ocular isolates during a period of 15 years. Am J Ophthalmol. 2004; 137:43–51.

31. Solomon R, Donnenfeld ED, Perry HD, et al. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology. 2005; 112:466–9.

32. Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol. 2002; 133:463–6.
33. Kim BK, Lee DW, Cho NC, You IC. Clinical aspect and prognosis of Staphylococcus epidermidis keratitis. J Korean Ophthalmol Soc. 2011; 52:14–22.
34. Jang YS, Hahn YH. Epidemiology of Staphylococcus epidermidis keratitis. J Korean Ophthalmol Soc. 2002; 43:665–71.
35. Vajpayee RB, Dada T, Saxena R, et al. Study of the first contact management profile of cases of infectious keratitis: a hospital-based study. Cornea. 2000; 19:52–6.

36. Ong SJ, Huang YC, Tan HY, et al. Staphylococcus aureus keratitis: a review of hospital cases. PLoS One. 2013; 8:e80119.

37. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008; 27:22–7.

38. Hahn YH, Hahn TW, Tchah H, et al. Epidemiology of infectious keratitis (II): a multi-center study. J Korean Ophthalmol Soc. 2001; 42:247–65.
40. Parmar P, Salman A, Kalavathy CM, et al. Pneumococcal keratitis: a clinical profile. Clin Experiment Ophthalmol. 2003; 31:44–7.

41. Charteris DG, Batterbury M, Armstrong M, Tullo AB. Suppurative keratitis caused by Streptococcus pneumoniae after cataract surgery. Br J Ophthalmol. 1994; 78:847–9.

42. Ormerod LD, Hertzmark E, Gomez DS, et al. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology. 1987; 94:1322–33.
43. Scott IU, Loo RH, Flynn HW Jr, Miller D. Endophthalmitis caused by Enterococcus faecalis: antibiotic selection and treatment outcomes. Ophthalmology. 2003; 110:1573–7.
44. Busbee BG, Recchia FM, Kaiser R, et al. Bleb-associated endophthalmitis: clinical characteristics and visual outcomes. Ophthalmology. 2004; 111:1495–503. discussion 1503.
45. Lee SM, Lee JH. A case of Enterococcus faecalis endophthalmitis with corneal ulcer. Korean J Ophthalmol. 2004; 18:175–9.

46. Rau G, Seedor JA, Shah MK, et al. Incidence and clinical characteristics of Enterococcus keratitis. Cornea. 2008; 27:895–9.

47. Elitsur Y, Biedner BZ, Bar-Ziv J. Ethmoiditis, conjunctivitis, and orbital cellulitis due to Enterococcus infection. Clin Pediatr (Phila). 1984; 23:123.
48. Kim JT, Lee JK, Moon NJ, Cho HK. A case of Enterococcus faecalis endophthalmitis after phacoemulsification. J Korean Ophthalmol Soc. 2006; 47:1853–8.
49. Reynaud af Geijersstam A, Sorsa T, Stackelberg S, et al. Effect of E. faecalis on the release of serine proteases elastase and cathepsin G, and collagenase-2 (MMP-8) by human polymorphonuclear leukocytes (PMNs). Int Endod J. 2005; 38:667–77.

50. Peng CH, Cheng CK, Chang CK, Chen YL. Multiresistant Enterococci: a rare cause of complicated corneal ulcer and review of the literature. Can J Ophthalmol. 2009; 44:214–5.

51. Zirakzadeh A, Patel R. Vancomycin-resistant Enterococci: colonization, infection, detection, and treatment. Mayo Clin Proc. 2006; 81:529–36.

52. Maple PA, Hamilton-Miller JM, Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989; 1:537–40.

53. Hsiao CH, Chuang CC, Tan HY, et al. Methicillin-resistant Staphylococcus aureus ocular infection: a 10-year hospital-based study. Ophthalmology. 2012; 119:522–7.

54. Waring GO III, Bouchard CS. A matrix of pathologic responses in the cornea. Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. 3rd ed.Philadelphia: Elsevier;2011. 1:chap. 5.

55. Miedziak AI, Miller MR, Rapuano CJ, et al. Risk factors in microbial keratitis leading to penetrating keratoplasty. Ophthalmology. 1999; 106:1166–70. discussion 1171.

Figure 1.
Prevalence of Gram-positive bacterial isolates in total bacterial keratitis during 1998-2012. The p-value was calculated using chi-square test to com pare the distribution of the Gram-positive bacterial isolates between 2 periods. *Gram-positive bacterial isolates significantly decreased between 2 periods.
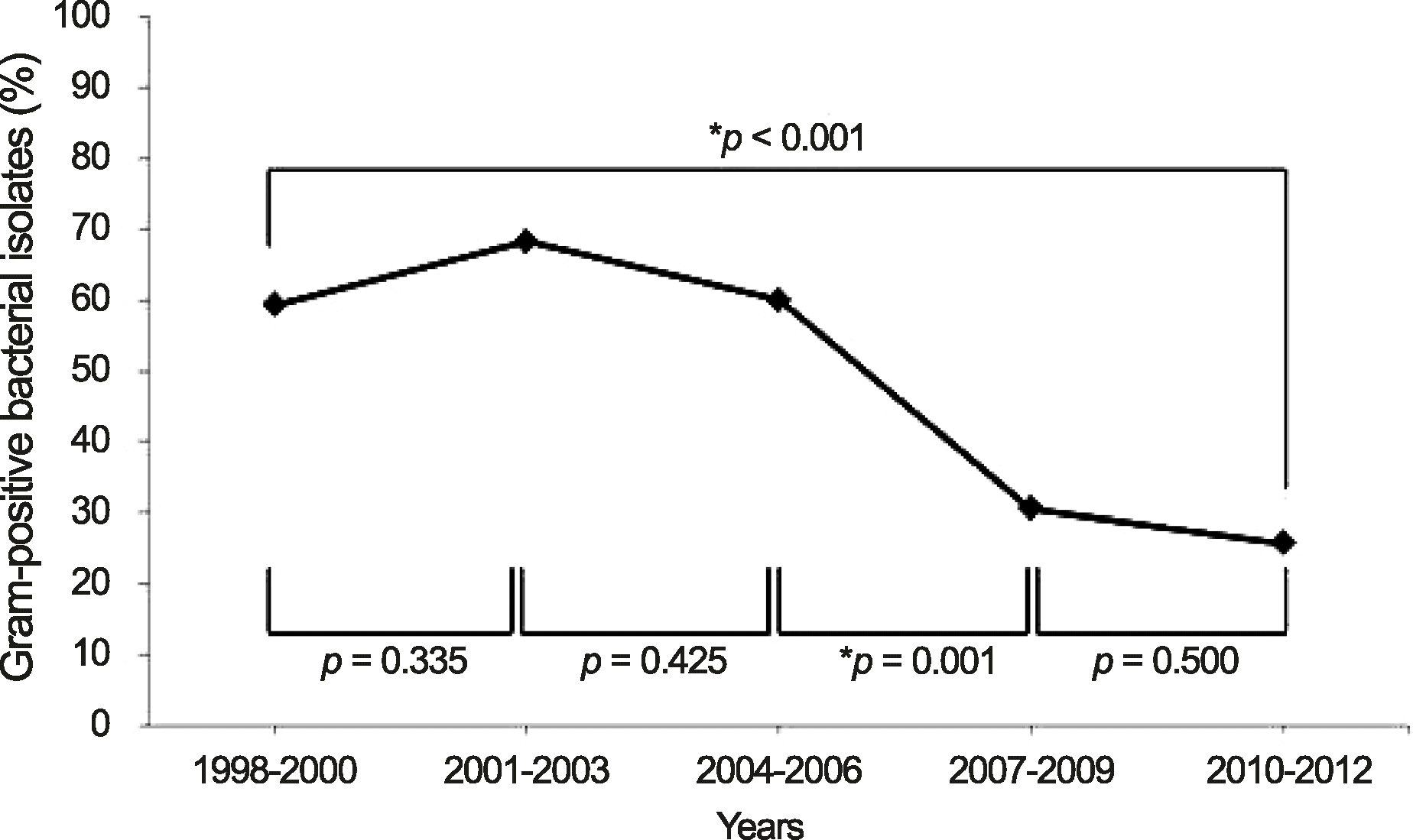
Figure 2.
Organisms and shifting trend in Gram-positive bacterial isolates during 1998-2012. *Six eyes had mixed infection of 2 Gram-positive bacterial species (S. epidermidis & S. aureus (2 eyes), S. epidermidis & E. feacalis, S. epidermidis & S. sanguis, S. aureus & E. feacalis, S. dysgalactiae & S. pyogenes); †The p-value was calculated using Spearman rank correlation coefficient to compare the distribution of the bacterial isolates for 15 years. CNS = coagulase-negative Staphylococcus.
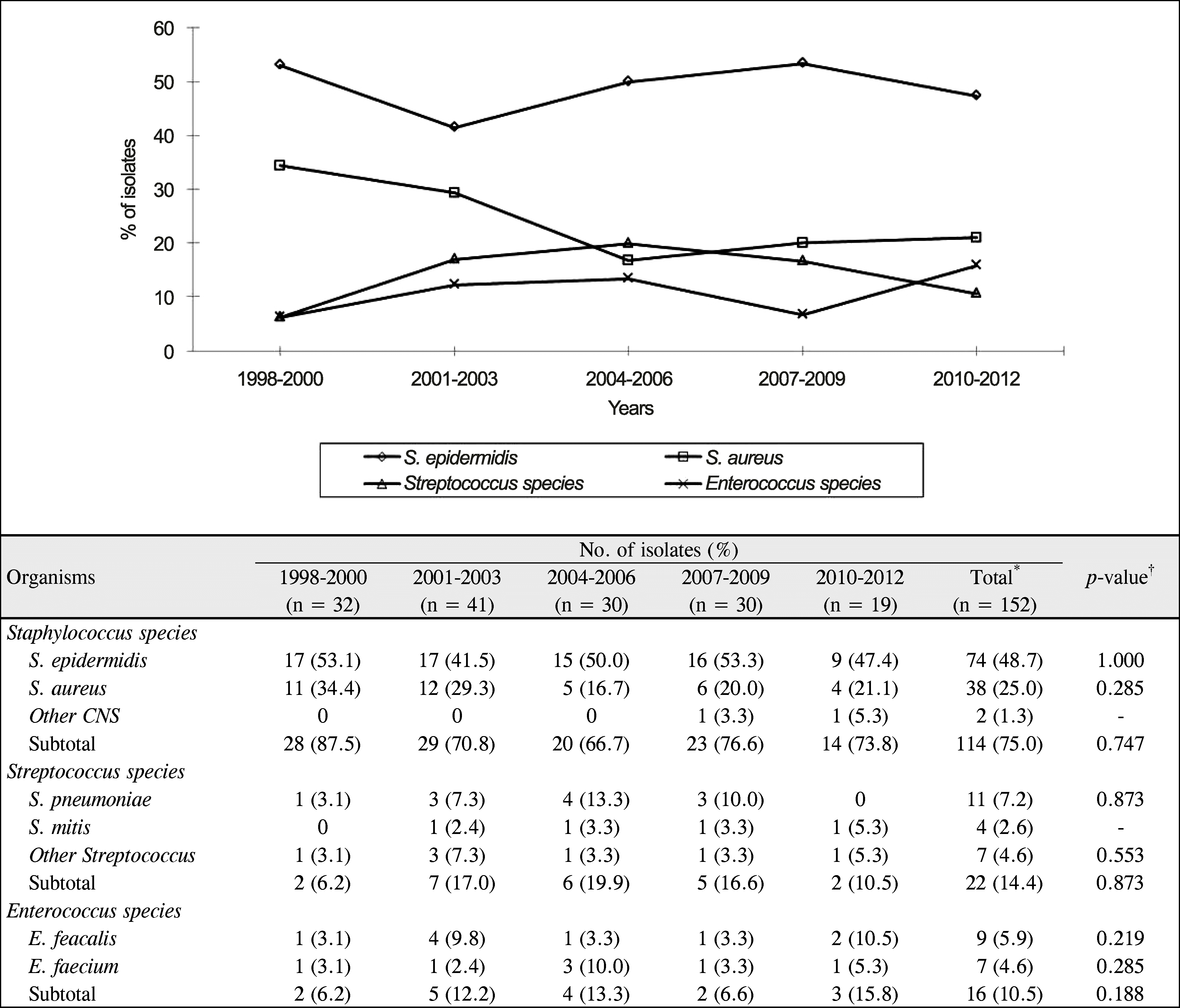
Figure 3.
Trends in antimicrobial resistance of Gram-positive bacterial isolates. *The resistance of gentamicin decreased significantly (p = 0.01, Spearman rank correlation coefficient); †N1 = number of isolates with resistance; N2 = number of tested isolates; ‡The p-value was calculated using Spearman rank correlation coefficient to compare the distribution of the antimicrobial resistance for 15 years; §Total value of fluoroquinolone: ciprofloxacin (n = 70), norfloxacin (n = 21), levofloxacin (n = 25), and moxifloxacin (n = 21).
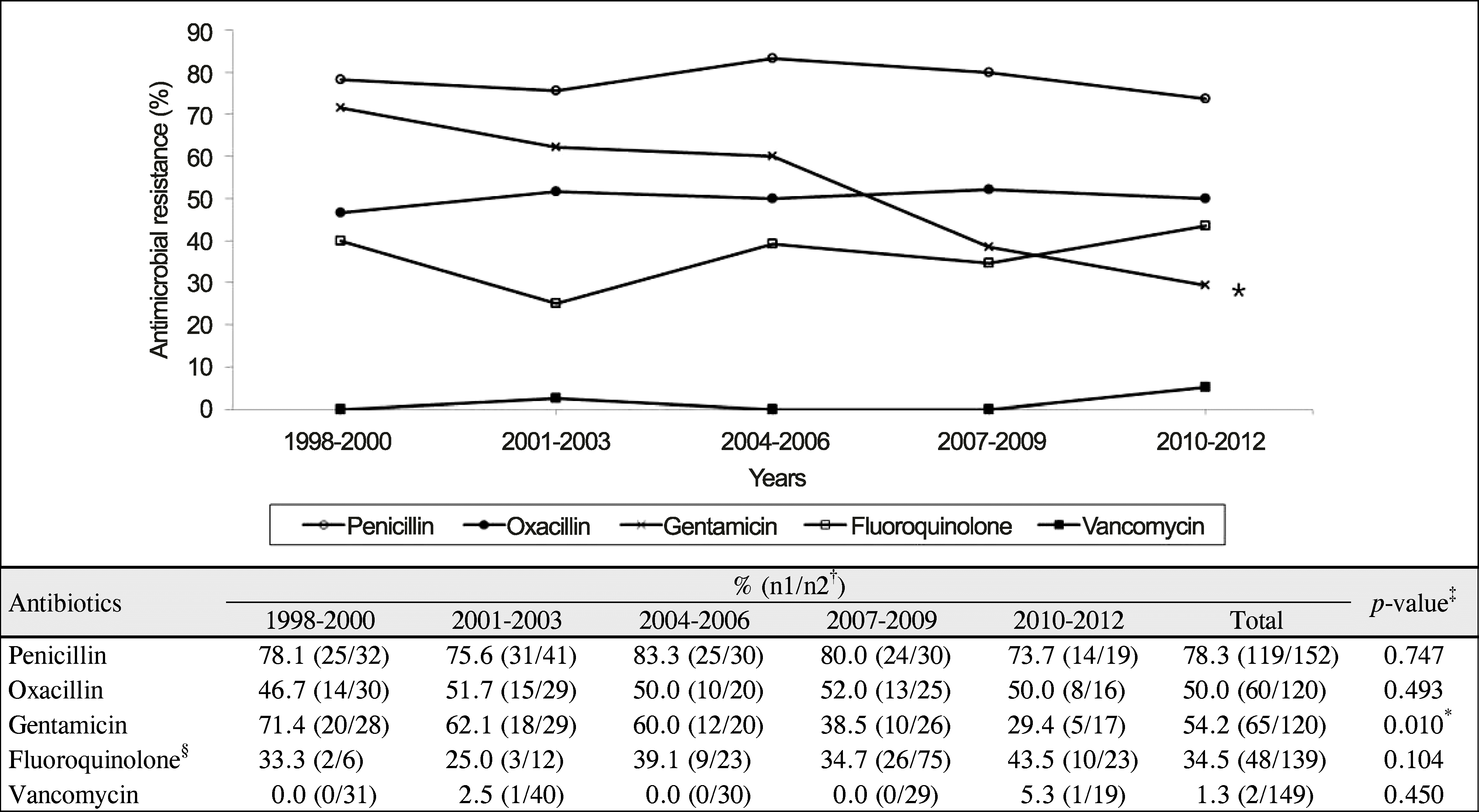
Figure 4.
Trends in antimicrobial resistance to oxacillin in S. epidermidis and S. aureus. Oxacillin resistance of S. epidermidis was significantly higher than that of S. aureus (p = 0.001, Chi-square test).
Table 1.
Demographics of Gram-positive bacterial keratitis according to the isolated microorganisms
|
No. of cases (%) |
|||||
|---|---|---|---|---|---|
| Characteristics | All case | S. epidermidis | S. aureus | Strepto. spp. | Entero. spp. |
| (n = 146) | (n = 74) | (n = 38) | (n = 22) | (n = 16) | |
| Sex (M:F) | 1.11:1 | 1.24:1 | 1.24:1 | 1:1 | 1:1 |
| Age (years) | |||||
| 60≤ | 68 (46.6) | 28 (37.8)* | 20 (52.6) | 15 (68.2) | 8 (50.0) |
| 40-59 | 44 (30.1) | 29 (39.2)* | 10 (26.3) | 2 (9.1) | 4 (25.0) |
| 20-39 | 22 (15.2) | 13 (17.6)* | 4 (10.5) | 2 (9.1) | 3 (18.8) |
| <20 | 12 (8.2) | 4 (5.4)* | 4 (10.5) | 3 (13.6) | 1 (6.3) |
| Seasonal distribution | |||||
| Spring (Mar-May) | 55 (37.7) | 28 (37.8) | 12 (31.6) | 9 (40.9) | 7 (43.8) |
| Summer (Jun-Aug) | 27 (18.5) | 13 (17.6) | 10 (26.3) | 2 (9.1) | 3 (18.8) |
| Autumn (Sep-Nov) | 30 (20.5) | 14 (18.9) | 6 (15.8) | 7 (31.8) | 3 (18.8) |
| Winter (Dec-Feb) | 34 (23.3) | 19 (25.7) | 10 (26.3) | 4 (18.2) | 3 (18.8) |
| Predisposing factors† | |||||
| Trauma | 53 (36.3) | 31 (41.9) | 9 (23.7) | 9 (40.9) | 5 (31.3) |
| Previous ocular surface disease | 45 (30.8) | 21 (28.4) | 14 (36.8) | 7 (31.8) | 6 (37.5) |
| Previous ocular surgery | 27 (18.5) | 12 (16.2) | 8 (21.1) | 4 (18.2) | 3 (18.8) |
| Systemic disease | 42 (28.8) | 17 (23.0) | 13 (34.2) | 6 (27.3) | 6 (37.5) |
| Contact lens wear | 8 (5.5) | 5 (6.8) | 2 (5.3) | 1 (4.5) | 1 (6.3) |
Table 2.
Clinical characteristics at initial presentation, epithelial healing time, surgical treatment, and visual outcome of Gram-positive bacterial keratitis according to the isolated microorganisms
|
No. of cases (%) |
|||||
|---|---|---|---|---|---|
| Characteristics | All cases | S. epidermidis | S. aureus | Strepto. spp. | Entero. spp. |
| (n = 146) | (n = 74) | (n = 38) | (n = 22) | (n = 16) | |
| Corneal lesion | |||||
| Location† | |||||
| Central | 91 (62.3) | 42 (56.8) | 23 (60.5) | 17 (77.3) | 12 (75.0) |
| Marginal | 55 (37.7) | 32 (43.2) | 15 (39.5) | 5 (22.7) | 4 (25.0) |
| Size | |||||
| <5 mm2 | 89 (61.0) | 52 (68.4) | 23 (60.5) | 10 (45.5) | 8 (50.0) |
| ≥5 mm2 | 57 (39.0) | 24 (31.6) | 15 (39.5) | 12 (54.5) | 8 (50.0) |
| Hypopyon | |||||
| No | 105 (71.9) | 56 (76.7) | 26 (68.4) | 14 (63.6) | 11 (68.8) |
| Yes | 41 (28.1) | 18 (24.3) | 12 (31.6) | 8 (36.4) | 5 (31.3) |
| Epithelial healing time‡ (n = 119) | 10.8 ± 11.1 | 8.8 ± 11.1* | 11.8 ± 8.6 | 13.6 ± 11.9 | 15.9 ± 14.4 |
| Surgical treatment | 22 (15.1) | 11 (14.9) | 7 (18.4) | 4 (18.2) | 4 (25.0) |
| Initial BCVA (Snellen acuity) | |||||
| <0.1 | 68 (46.6) | 29 (39.2) | 19 (50.0) | 14 (63.6) | 8 (50.0) |
| 0.1-0.5 | 54 (37.0) | 33 (44.6) | 14 (36.8) | 4 (18.2) | 6 (37.5) |
| 0.6-1.0 | 24 (16.4) | 12 (16.2) | 5 (13.2) | 4 (18.2) | 2 (12.5) |
| Final BCVA (Snellen acuity) | |||||
| <0.1 | 44 (30.1) | 16 (21.7)* | 14 (36.8) | 10 (45.5) | 8 (50.0) |
| 0.1-0.5 | 39 (26.7) | 23 (31.1)* | 10 (26.3) | 5 (22.7) | 2 (12.5) |
| 0.6-1.0 | 63 (43.2) | 35 (47.3)* | 14 (36.8) | 7 (31.8) | 6 (37.5) |
Strepto. spp. = Streptococcus species; Entero. spp. = Enterococcus species; BCVA = best corrected visual acuity.
* The p-value was < 0.05, which was calculated for comparison of proportions with all other groups by independent t-test or chi-square test;
Table 3.
Comparisons of epidemiologic and clinical features and treatment outcome between MSS and MRS
| Characteristics |
No. of cases (%) |
p-value‡ | |
|---|---|---|---|
| MSS (n = 56) | MRS (n = 56) | ||
| Sex (M:F) | 1.24:1 | 1.24:1 | 1.000 |
| Age (years) | 0.399 | ||
| 60≤ | 21 (37.5) | 27 (48.2) | |
| 40-59 | 21 (37.5) | 18 (32.1) | |
| 20-39 | 11 (19.6) | 6 (10.7) | |
| <20 | 3 (5.4) | 5 (8.9) | |
| Seasonal distribution | 0.377 | ||
| Spring (Mar-May) | 17 (30.4) | 23 (41.1) | |
| Summer (Jun-Aug) | 14 (25.0) | 9 (16.1) | |
| Autumn (Sep-Nov) | 12 (21.4) | 8 (14.3) | |
| Winter (Dec-Feb) | 13 (23.2) | 16 (28.6) | |
| Predisposing factors | |||
| Trauma | 15 (26.8) | 25 (44.6) | 0.049 |
| Previous ocular surface disease | 18 (32.1) | 17 (30.4) | 0.838 |
| Previous ocular surgery | 9 (16.1) | 11 (19.6) | 0.622 |
| Systemic disease | 13 (23.2) | 17 (30.4) | 0.393 |
| Contact lens wear | 5 (8.9) | 2 (1.8) | 0.438§ |
| Corneal lesion | |||
| Location* | 0.566 | ||
| Central | 34 (60.7) | 31 (55.4) | |
| Marginal | 22 (39.3) | 25 (44.6) | |
| Size (mm2) | 0.321 | ||
| <5 | 34 (60.7) | 39 (69.6) | |
| ≥5 | 22 (39.3) | 17 (30.4) | |
| Hypopyon | 0.670 | ||
| No | 40 (71.4) | 42 (75.0) | |
| Yes | 16 (28.6) | 14 (25.0) | |
| Epithelial healing time (n = 90)† | 11.1 ± 10.8 | 8.6 ± 9.9 | 0.259Π |
| Surgical treatment | 12 (21.4) | 6 (10.7) | 0.123 |
| Initial BCVA (Snellen acuity) | 0.589 | ||
| <0.1 | 25 (44.6) | 23 (41.1) | |
| 0.1-0.5 | 24 (42.9) | 22 (39.3) | |
| 0.6-1.0 | 7 (12.5) | 11 (19.6) | |
| Final BCVA (Snellen acuity) | 0.165 | ||
| <0.1 | 19 (33.9) | 12 (21.4) | |
| 0.1-0.5 | 12 (21.4) | 20 (35.7) | |
| 0.6-1.0 | 25 (44.6) | 24 (42.9) | |
MSS = methicillin-sensitive Staphylococcus; MRS = methicillin-resistant Staphylococcus; BCVA = best corrected visual acuity.
* ‘Central’ is located within 1/2 radius from the center of the cornea, ‘Marginal’ is located within 1/2 radius from the limbus;
Table 4.
Risk factors for poor visual outcome and surgical treatment in Gram-positive bacterial keratitis (univariate logistic regression analysis)
|
Poor visual outcome |
Surgical treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Factor | No. of eyes (%)* | OR | 95% CI | p-value | No. of eyes (%)† | OR | 95% CI | p-value |
| (n = 45) | (n = 22) | |||||||
| Sex | ||||||||
| Female | 19/69 (27.5) | 1.00 | 10/69 (14.5) | 1.00 | ||||
| Male | 26/77 (33.8) | 1.34 | 0.66-2.72 | 0.416 | 12/77 (15.6) | 1.09 | 0.43-2.70 | 0.854 |
| Cultured organisms | 0.061 | 0.802 | ||||||
| S. epidermidis | 16/74 (21.6) | 1.00 | 11/74 (14.9) | 1.00 | ||||
| S. aureus | 14/38 (36.8) | 2.04 | 0.85-4.92 | 0.109 | 8/38 (21.1) | 1.29 | 0.46-3.66 | 0.628 |
| Strepto. spp. | 11/22 (50.0) | 2.96 | 1.05-8.39 | 0.041 | 4/22 (18.2) | 1.27 | 0.36-4.48 | 0.707 |
| Entero. spp. | 7/16 (43.8) | 3.62 | 1.11-11.86 | 0.033 | 3/16 (18.8) | 1.91 | 0.52-7.01 | 0.330 |
| Age (years) | ||||||||
| <60 | 13/78 (16.7) | 1.00 | 8/78 (10.3) | 1.00 | ||||
| ≥60 | 32/68 (47.1) | 4.44 | 2.07-9.53 | 0.000 | 14/68 (20.6) | 2.27 | 0.89-5.80 | 0.087 |
| Previous ocular surface disease | ||||||||
| No or unknown | 26/101 (25.7) | 1.00 | 12/101 (11.9) | 1.00 | ||||
| Yes | 19/45 (42.2) | 2.10 | 1.01-4.42 | 0.049 | 10/45 (22.2) | 2.12 | 0.84-5.35 | 0.112 |
| Previous ocular surgery | ||||||||
| No or unknown | 34/119 (28.6) | 1.00 | 15/119 (12.6) | 1.00 | ||||
| Yes | 11/27 (40.7) | 1.71 | 0.72-4.08 | 0.220 | 7/27 (25.9) | 2.43 | 0.88-6.71 | 0.087 |
| Ocular trauma history | ||||||||
| No or unknown | 31/93 (33.3) | 1.00 | 15/93 (16.1) | 1.00 | ||||
| Yes | 14/53 (26.4) | 0.71 | 0.34-1.52 | 0.385 | 7/53 (13.2) | 0.79 | 0.30-2.08 | 0.636 |
| Systemic disease | ||||||||
| No or unknown | 27/104 (26.0) | 1.00 | 14/104 (13.5) | 1.00 | ||||
| Yes | 18/42 (42.9) | 2.13 | 1.01-4.54 | 0.048 | 8/42 (19.0) | 1.51 | 0.59-3.93 | 0.395 |
| Symptom to treatment interval | ||||||||
| ≤1 (week) | 29/111 (26.1) | 1.00 | 15/111 (13.5) | 1.00 | ||||
| >1 (week) | 16/35 (45.7) | 2.38 | 1.08-5.24 | 0.031 | 7/35 (20.0) | 1.60 | 0.59-4.31 | 0.353 |
| Location of corneal lesion | ||||||||
| Marginal | 9/56 (20.0) | 1.00 | 6/56 (10.7) | 1.00 | ||||
| Central | 36/90 (40.0) | 3.48 | 1.52-7.97 | 0.003 | 16/90 (17.8) | 1.80 | 0.66-4.92 | 0.251 |
| Size of epithelial defect (mm2) | ||||||||
| <5 | 12/89 (13.5) | 1.00 | 7/89 (7.8) | 1.00 | ||||
| ≥5 | 33/57 (57.9) | 8.82 | 3.95-19.72 | <0.001 | 15/57 (26.8) | 4.34 | 1.64-11.47 | 0.003 |
| Hypopyon | ||||||||
| No | 15/105 (14.3) | 1.00 | 4/105 (3.8) | 1.00 | ||||
| Yes | 30/41 (73.2) | 16.36 | 6.78-39.49 | <0.001 | 18/41 (43.9) | 19.76 | 6.11-63.94 | <0.001 |
| Initial BCVA | ||||||||
| ≥0.1 | 2/78 (2.6) | 1.00 | 1/78 (1.3) | 1.00 | ||||
| <0.1 | 43/68 (63.2) | 65.36 | 14.76-289.44 | <0.001 | 21/68 (30.9) | 34.40 | 4.48-264.23 | 0.001 |
| Epithelial healing time (days) | ||||||||
| <10 | 3/73 (4.1) | 1.00 | ||||||
| ≥10 | 42/73 (57.5) | 31.61 | 9.10-109.82 | <0.001 | ||||
Table 5.
Risk factors for poor visual outcome and surgical treatment in Gram-positive bacterial keratitis (multivariate logistic regression analysis*)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download