Abstract
Purpose
To investigate the effect of L-ascorbic acid (LAA) on oxidative-stress-induced cellular senescence in trabecular meshwork (TM) cells.
Methods
Primarily cultured human TM cells were exposed to 0, 10, or 100 μM hydrogen peroxide for 7 days with or without co-exposure of LAA. Cellular survival and nitrite production were assessed with MTT and Griess assays, respectively. SA-β-gal staining was performed to quantify cellular senescence.
Results
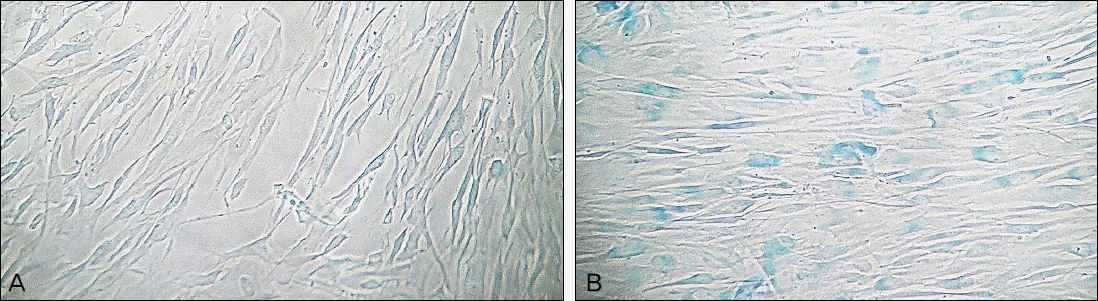
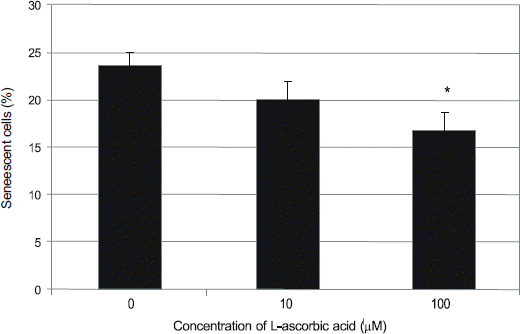
Hydrogen peroxide decreased cellular survival, accompanied by decreased nitric oxide (NO) production. These decreases of cellular survival and NO production were abolished by co-exposure of 100 μM LAA. Analysis of SA-β-gal staining revealed that LAA inhibited hydrogen peroxide-induced cellular senescence by 6.8% (p < 0.05).
References
1. Saccà SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005; 123:458–63.
2. von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000; 908:99–110.

3. BECKER B. Chemical composition of human aqueous humor; effects of acetazoleamide. AMA Arch Ophthalmol. 1957; 57:793–800.
4. Erb C, Nau-Staudt K, Flammer J, Nau W. Ascorbic acid as a free radical scavenger in porcine and bovine aqueous humour. Ophthalmic Res. 2004; 36:38–42.

5. Heller R, Münscher-Paulig F, Gräbner R, Till U. L-Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem. 1999; 274:8254–60.

6. Kim JW. Ascorbic acid enhances nitric oxide production in trabecular meshwork cells. Korean J Ophthalmol. 2005; 19:227–32.

7. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, path-ophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.
8. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.

9. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.
10. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eye. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.

11. Meyer P, Champion C, Schlötzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.

12. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994; 58:99–105.

13. Wang RF, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.

14. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.
15. Matsuo T. Basal nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.

16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

17. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–8.

18. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.
19. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.
20. Ross R. Atherosclerosisan inflammatory disease. N Engl J Med. 1999; 340:115–26.
21. May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000; 28:1421–9.

22. Huang A, Vita JA, Venema RC, Keaney JF. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intra-cellular tetrahydrobiopterin. J Biol Chem. 2000; 275:17399–406.

23. Heller R, Unbehaun A, Schellenberg B, et al. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001; 276:40–7.

24. Haefliger IO, Dettmann E, Liu R, et al. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol. 1999; 43:S51–8.

25. Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004; 24:413–20.

26. Brüne B, Von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.

27. Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000; 87:540–2.

28. Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol. 1999; 180:182–9.

29. Kurz DJ, Decary S, Hong Y, et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004; 117((Pt 11)):2417–26.

30. von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000; 908:99–110.

31. Furumoto K, Inoue E, Nagao N, et al. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998; 63:935–48.

Figure 1.
Effect of hydrogen peroxide on the survival of human trabecular meshwork cells. Exposure to 100 μM hydrogen peroxide decreased cellular proliferation significantly compared to non-exposed control (*p < 0.05).
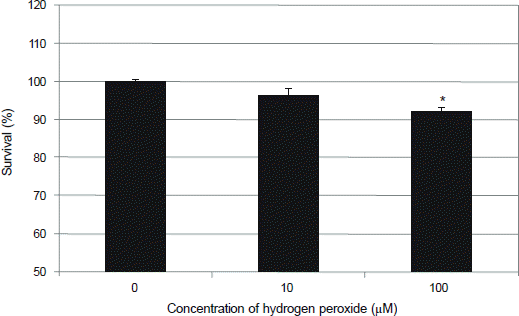
Figure 2.
Effect of L-ascorbic acid on the survival of human trabecular meshwork cells against the hydrogen peroxide-induced oxidative stress. 100 μM hydrogen peroxide decreased cellular survival significantly (*p < 0.05) which were abolished by L-ascorbic acid (p > 0.05).
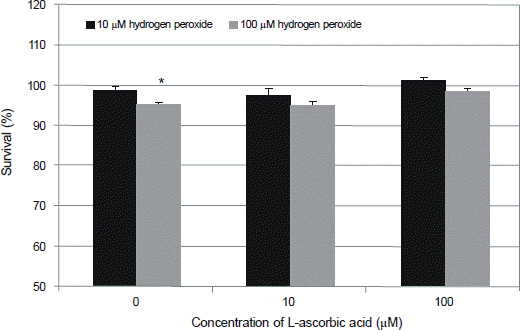
Figure 3.
Effect of hydrogen peroxide on the production of nitric oxide (NO) in primarily cultured human trabecular meshwork cells. Exposure to 100 μM hydrogen peroxide decreased production of NO significantly compared to non-exposed control (*p < 0.05).
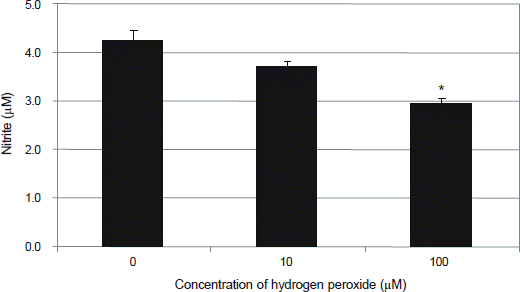
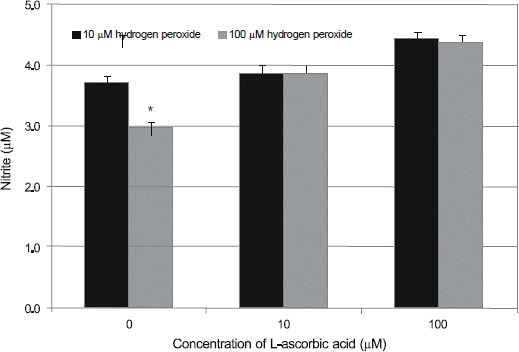
Figure 4.
Effect of L-ascorbic acid on the production of nitric oxide (NO) in human trabecular meshwork cells against the hydrogen peroxide-induced oxidative stress. L-ascorbic acid increased production of NO compared to non-exposed control (*p < 0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download