Abstract
Purpose
To compare clinical outcomes after intravitreal injection of triamcinolone acetonide or bevacizumab for the treatment of macular edema secondary to branch retinal vein occlusion.
Methods
Sixty-six patients received an intravitreal injection of either triamcinolone acetonide or bevacizumab. Patients were retrospectively reviewed. Thirty-three out of 66 patients were treated with an intravitreal injection of triamcinolone acetonide, while the other 33 patients received a bevacizumab injection. All patients underwent a visual acuity test, optical coherence tomography imaging and ophthalmoscopic examination throughout the follow-up.
Results
In the triamcinolone group, central macular thickness (CMT) decreased from 496.69±153.01 μ m at baseline to 313.06±150.14 μ m at the six-month follow-up visit, while in the bevacizumab group, CMT decreased from 441.30 ± 185.79 μ m to 295.67 ± 188.80 μ m (p<0.05). In the triamcinolone group, best-corrected visual acuity (BCVA) improved from logMAR 0.92 ± 0.70 at baseline to logMAR 0.53 ± 0.43 at the six-month follow-up visit, and in the bevacizumab group, BCVA improved from logMAR 0.74 ± 0.47 to logMAR 0.34 ± 0.33 (p<0.05).
References
1. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984; 98:271–82.
2. Duff IF, Falls HF, Linman JW. Anticoagulant therapy in occlusive vascular disease of the retina. AMA Arch Ophthalmol. 1951; 46:601–17.

3. Michels RG, Gass JD. The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol. 1974; 78:OP166–77.

4. Han DP, Mieler WF, Burton TC. Submacular fibrosis after photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992; 113:513–21.

5. Striph GG, Hart WM Jr, Olk RJ. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology. 1988; 95:1673–9.
6. Lewis H, Schachat AP, Haimann MH, et al. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology. 1990; 97:503–10. discussion 510-1.

7. Jonas JB, Akkoyun I, Kamppeter B, et al. Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide. Eye. 2005; 19:65–71.

8. Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007; 27:419–25.

9. Yang HN, Kim YJ, Kim JC, Shyn KH. Clinical Evaluation for Branch Retinal Vein Occlusion. J Korean Ophthalmol Soc. 1992; 33:599–604.
10. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008; 33:111–31.

11. Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001; 108:765–72.

12. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

13. Andrade RE, Muccioli C, Farah ME, et al. Intravitreal triamcinolone in the treatment of serous retinal detachment in Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 2004; 137:572–4.

14. Goff MJ, Jumper JM, Yang SS, et al. Intravitreal triamcinolone acetonide treatment of macular edema associated with central retinal vein occlusion. Retina. 2006; 26:896–901.

15. Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005; 25:851–5.

16. Krepler K, Ergun E, Sacu S, et al. Intravitreal triamcinolone acetonide in patients with macular oedema due to branch retinal vein occlusion: a pilot study. Acta Ophthalmol Scand. 2005; 83:600–4.

17. Pai SA, Shetty R, Vijayan PB, et al. Clinical, anatomic, and electro-physiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol. 2007; 143:601–6.

18. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.
19. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007; 114:743–50.
20. Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
21. Gunduz K, Bakri SJ. Intravitreal bevacizumab for macular oedema secondary to branch retinal vein occlusion. Eye (Lond). 2008; 22:1168–71.
22. Jaissle GB, Leitritz M, Gelisken F, et al. One-year results after intravitreal bevacizumab therapy for macular edema secondary to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2009; 247:27–33.

23. Yepremyan M, Wertz FD, Tivnan T, et al. Early treatment of cystoid macular edema secondary to branch retinal vein occlusion with intravitreal triamcinolone acetonide. Ophthalmic Surg Lasers Imaging. 2005; 36:30–6.

25. Ota M, Tsujikawa A, Murakami T, et al. Foveal photoreceptor layer in eyes with persistent cystoid macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2008; 145:273–80.

26. McCuen BW 2nd, Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981; 91:785–8.

27. Kivilcim M, Peyman GA, El-Dessouky ES, et al. Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. Ophthalmic Surg Lasers. 2000; 31:474–8.

28. Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006; 26:257–61.

29. Vasconcelos-Santos DV, Nehemy PG, Schachat AP, Nehemy MB. Secondary ocular hypertension after intravitreal injection of 4 mg of triamcinolone acetonide: incidence and risk factors. Retina. 2008; 28:573–80.
Figure 1.
The changes of mean visual acuity (logMAR) of triamcinolone group and bevacizumab group in patients with macular edema associated with branch retinal vein occlusion.
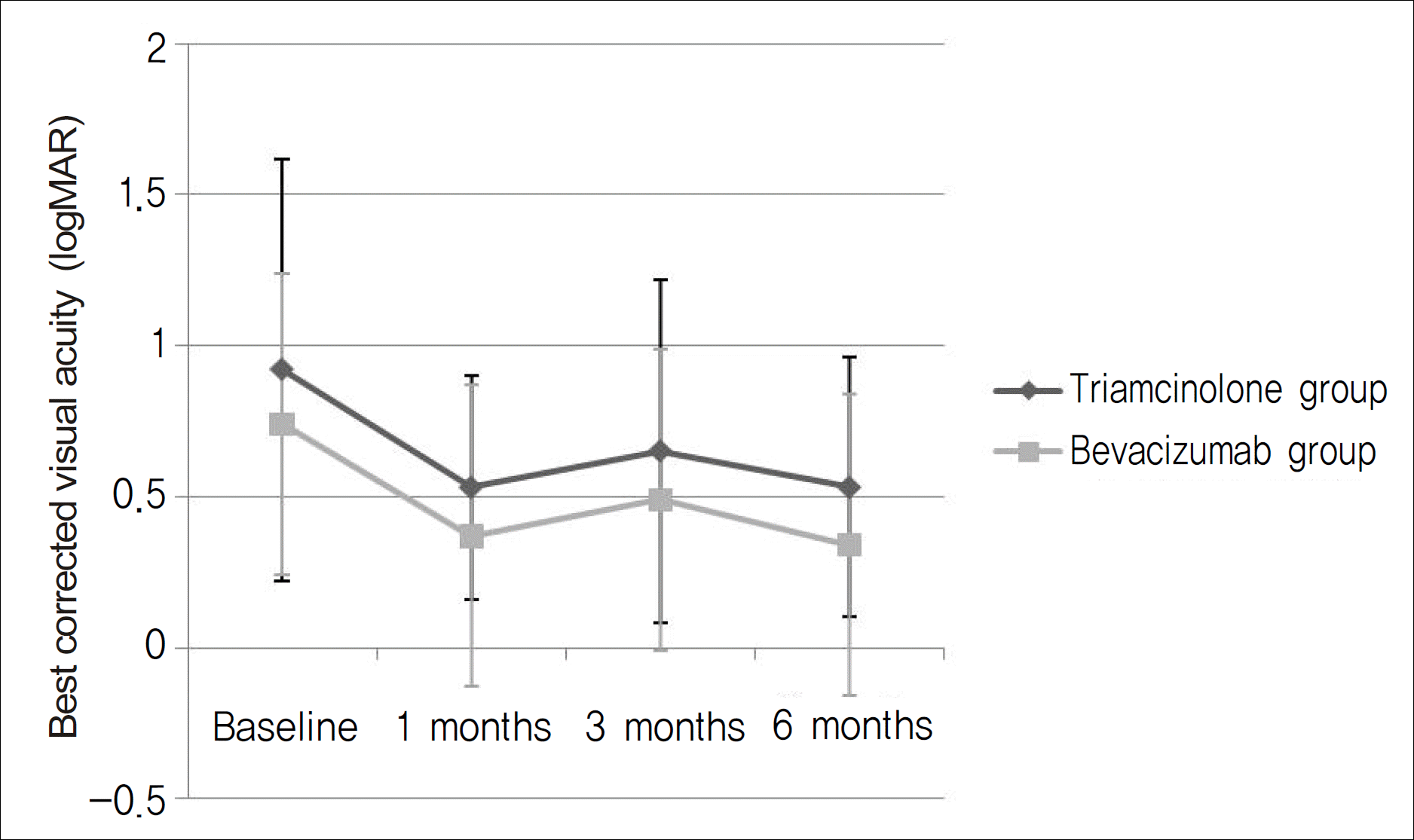
Figure 2.
The changes of central macular thickness of triamcinolone group and bevacizumab group in patients with macular edema associated with branch retinal vein occlusion.
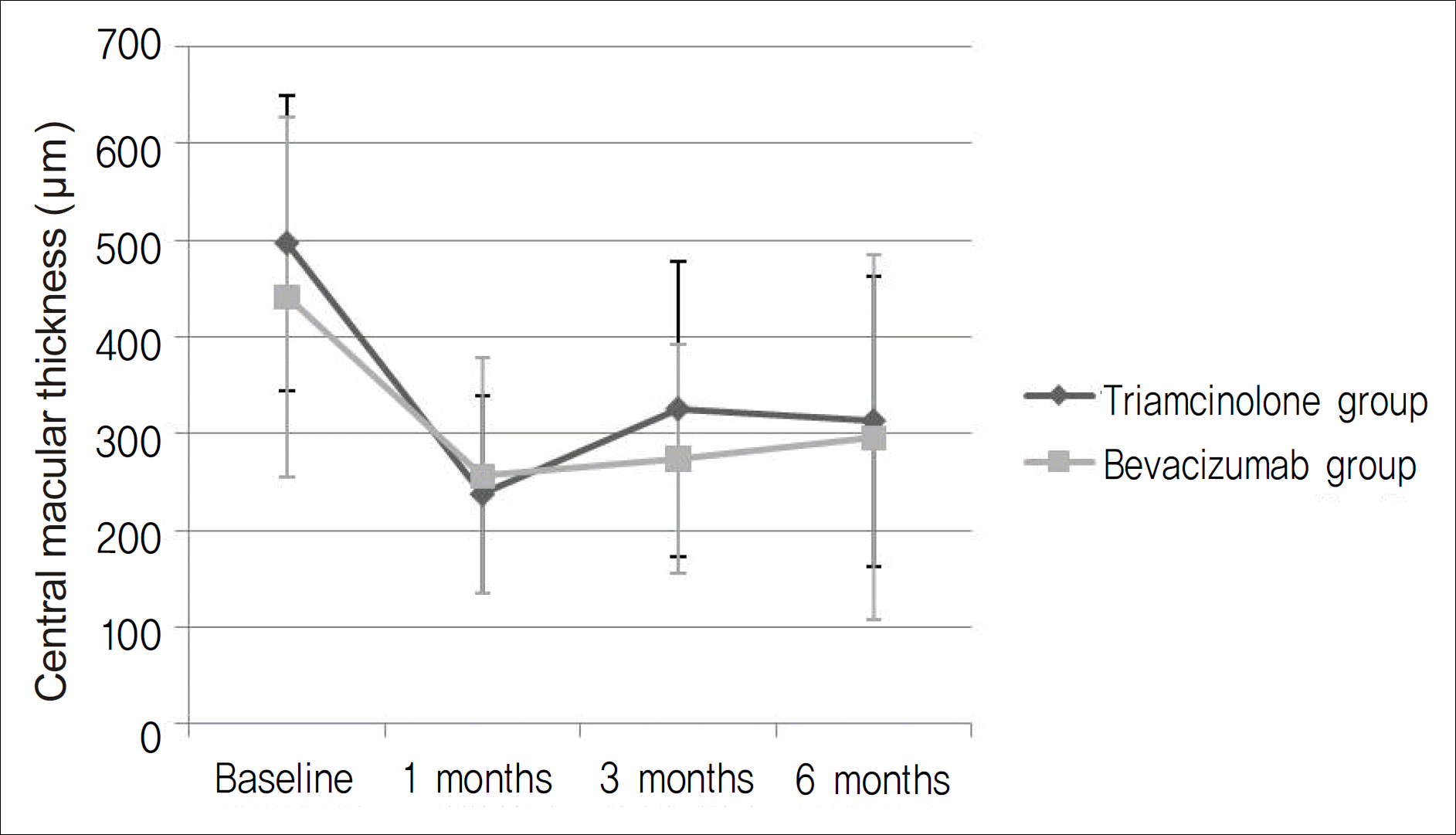
Table 1.
Demographic data of patients
| Triamcinolone group (n=33) | Bevacizumab group (n=33) | p value | |
|---|---|---|---|
| Age (yr) | 58.15 ± 10.67 | 57.61 ± 10.01 | 0.83 |
| Gender (M:F) | 12:21 | 15:18 | 0.68 |
| Baseline BCVA* (logMAR) | 0.92 ± 0.70 | 0.74 ± 0.47 | 0.32 |
| Baseline CMT† (μ m) | 496.69 ± 153.01 | 441.30 ± 185.79 | 0.21 |
Table 2.
Change of visual acuity and central macular thickness
|
Triamcinolone group (n=33) |
Bevacizumab group (n=733) |
|||
|---|---|---|---|---|
| BCVA* (logMAR) | CMT† (μ m) | BCVA* (logMAR) | CMT† (μ m) | |
| Baseline | 0.92 ± 0.70 | 496.69 ± 153.01 | 0.74 ± 0.47 | 441.30 ± 185.79 |
| 1 month | 0.53 ± 0.37‡ | 237.19 ± 102.15‡ | 0.37 ± 0.38‡ | 256.08 ± 121.73‡ |
| 3 months | 0.65 ± 0.57‡ | 325.44 ± 152.32‡ | 0.49 ± 0.47‡ | 273.80 ± 118.15‡ |
| 6 months | 0.53 ± 0.43‡ | 313.06 ± 150.14‡ | 0.34 ± 0.33‡ | 295.67 ± 188.80‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download