Abstract
Purpose
To evaluate the biological effects and cytotoxicity of gel-type artificial tears on human corneal keratocytes and conjunctival cells in vitro.
Methods
Human corneal keratocytes and conjunctival epithelial cells were exposed to Soothe® and Systane® at variable concentrations. Evaluations were conducted through an MTT-based calorimetric assay to measure the metabolic activity and through a lactate dehydrogenase (LDH) assay to assess cellular damage. Apoptotic response was examined using fluorescent microscopy and flow cytometric analysis, and cellular morphologic results were evaluated with a transmission electron microscope.
Results
The inhibitory effects of corneal keratocyte and conjunctival cell proliferations increased at higher concentrations and longer exposure times to Soothe® and Systane®. The LDH titers increased after Soothe® exposure, but showed no significant difference after Systane® exposure. Soothe® and Systane® treatments both produced fluorescence, representing apoptotic cells. In flow cytometry, the maximal apoptotic response was observed for both types of artificial tears, although Systane® showed less edema, as well as reduced cytoplasmic and nuclear cell degeneration compared to those of Soothe®.
Conclusions
The apoptotic responses of Soothe® and Systane® are associated with inhibitory effects of human corneal keratocyte and conjunctival epithelial cell proliferations. To inhibit the cellular proliferation of human corneal keratocytes and conjunctival epithelial cells, Systane® may be less severe than Soothe® at higher concentrations and longer exposure times.
References
1. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995; 21:221–32.
2. Perry HD, Donnenfeld ED. Dry eye diagnosis and management in 2004. Curr Opin Ophthalmol. 2004; 15:299–304.

3. Smith RE. The tear film complex pathogenesis and emerging therapies for dry eyes. Cornea. 2005; 24:1–7.
4. Balaram M, Schaumberg DA, Dana RD. Efficacy and tolerability outcomes after punctual occlusion with silicone plugs in dry eye syndrome. Am J Ophthalmol. 2001; 131:30–6.
5. Isreb MA, Greiner JV, Korb DR, et al. Correlation of lipid layer thickness measurements with fluorescein tear film breakup time and Schirmer's test. Eye. 2003; 17:79–83.

6. Greiner JV, Glonek T. Treatment of dry eye with a metastable formulation containing lipids and dineric interfacial molecules. Presented at the American Academy of Ophthalmology Annual Meeting. 2004.
7. Korb DR, Scaffidi RC, Greiner JV, et al. The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom Vis Sci. 2005; 82:594–601.

8. Petricek I, Berta A, Higazy MT, et al. Hydroxypropyl-guar gellable lubricant eye drops for dry eye treatment. Expert Opin Pharmacother. 2008; 9:1431–6.

9. Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-guar gellable lubricant eye drop for the relief of dryness of the eye. Curr Eye Res. 2004; 28:55–62.

10. Ubels JL, Clousing DP, Van Haitsma TA, et al. Pre-clinical investigation of the efficacy of an artificial tear solution containing hy-droxypropyl-guar as a gelling agent. Curr Eye Res. 2004; 28:437–44.

11. Schein OD, Munoz B, Tielsch JM, et al. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997; 124:723–8.

12. Woon KC, Im SK, Park YG, et al. Tear film and ocular surface changes after umbilical cord serum therapy in dry eye syndrome. J Korean Ophthalmol Soc. 2005; 46:237–42.
13. Heo H, Kang IS, Wu MH, et al. Therapeutic effect of topical testos-terone gel in patients with dry eye syndrome. J Korean Ophthalmol Soc. 2006; 47:1259–65.
14. Trees GR, Tomlinson A. Effect of artificial tear solutions and saline on tear film evaporation. Optom Vis Sci. 1990; 67:886–90.

15. Korb DR, Scaffidi RC, Greiner JV, et al. The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom Vis Sci. 2005; 82:594–601.

16. Hartstein I, Khwarg S, Przydryga J. An open-label evaluation of HP-Guar gellable lubricant eye drops for the improvement of dry eye signs and symptoms in a moderate dry eye adult population. Curr Med Res Opin. 2005; 21:255–60.

17. Sall KN, Cohen SM, Christensen MT, et al. An evaluation of the efficacy of a cyclosporine-based dry eye therapy when used with marked artificial tears as supportive therapy in dry eye. Eye Contact Lens. 2006; 32:21–6.
18. Gifford P, Evans BJ, Morris J. A clinical evaluation of Systane. Contact Lens Anterior Eye. 2006; 29:31–40.

19. Adams J, Wilcox MJ, Trousdale MD, et al. Morphologic and physiologic effects of artificial tear formulations on corneal epithelial derived cells. Cornea. 1992; 11:234–41.

20. Berdy GJ, Abelson MB, Smith LM, et al. Preservative-free artificial tear preparations Assessment of corneal epithelial toxic effects. Arch Ophthalmol. 1992; 110:528–32.
21. Diebold Y, Herreras JM, Callejo S, et al. Carbomer-versus cellu-lose-based artificial-tear formulations: morphologic and toxico-logic effects on a corneal cell line. Cornea. 1998; 17:433–40.
22. Kasper K, Kremling C, Geerling G. Toxicity of a new moistening agent and preservative in vitro. Ophthalmologe. 2008; 105:557–62.
23. Adler R. Mechanism of photoreceptor death in retinal degeneration. From the cell biology of the 1990s to the ophthalmology or the 21st century. Arch Ophthalmol. 1996; 114:79–83.
24. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995; 267:1456–62.

25. Muller G, Kramer A. Biocompatibility of index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008; 61:1281–7.
Figure 1.
The absorption rate of the water-insolubale formazan dye in corneal keratocytes exposed in Soothe® and Systane® by a scanning spectrometer (ELISA reader). Metabolic activity of keratocytes was decreased, at the higher concentration and longer the exposure duration in both artificial tears. The survival rate of Systane® was higher value than that of Soothe®.
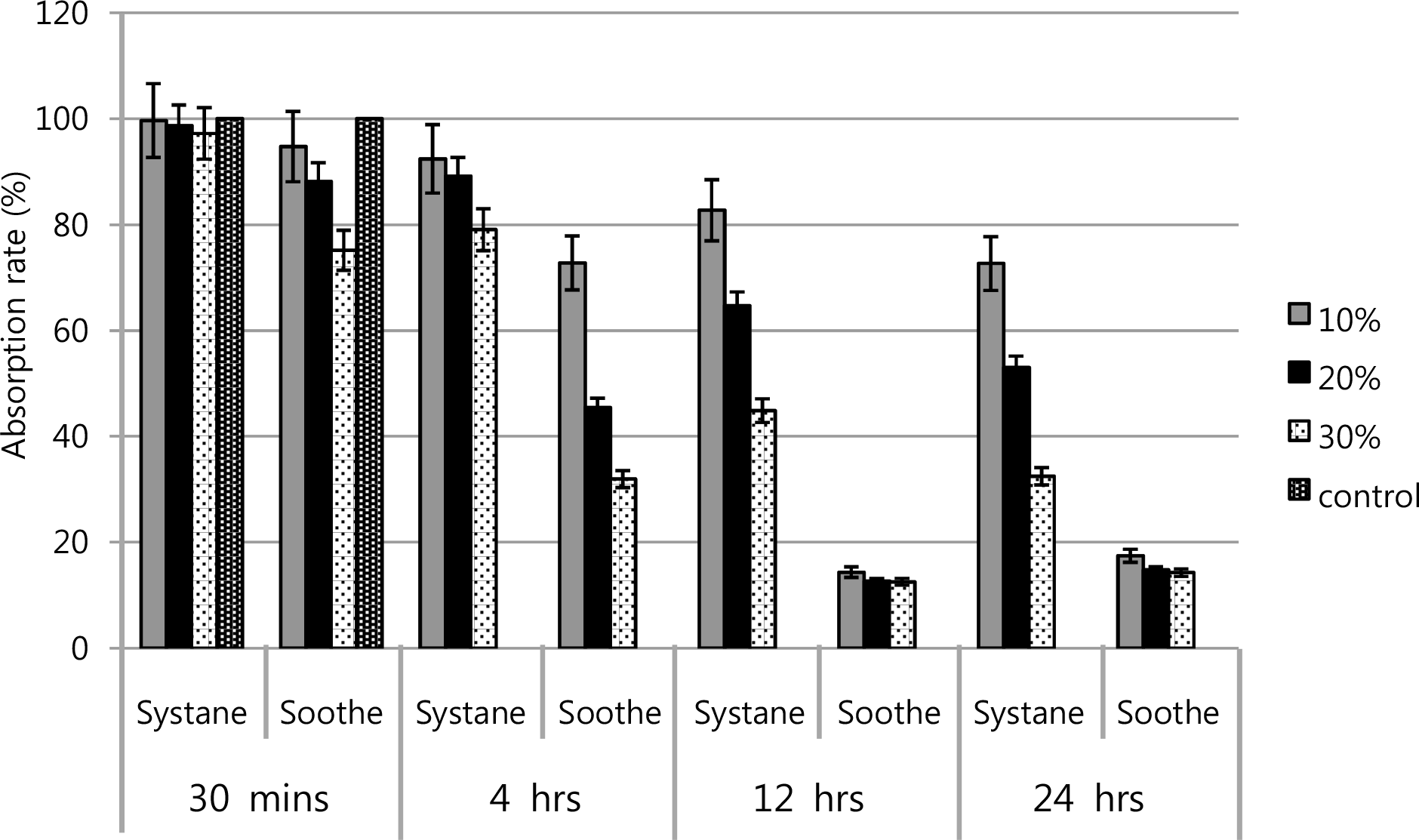
Figure 2.
Metabolic activities of cultured human conjunctival epithelial cells showed the dose-response and time-response. Soothe® has more inhibitory effect than Systane®.
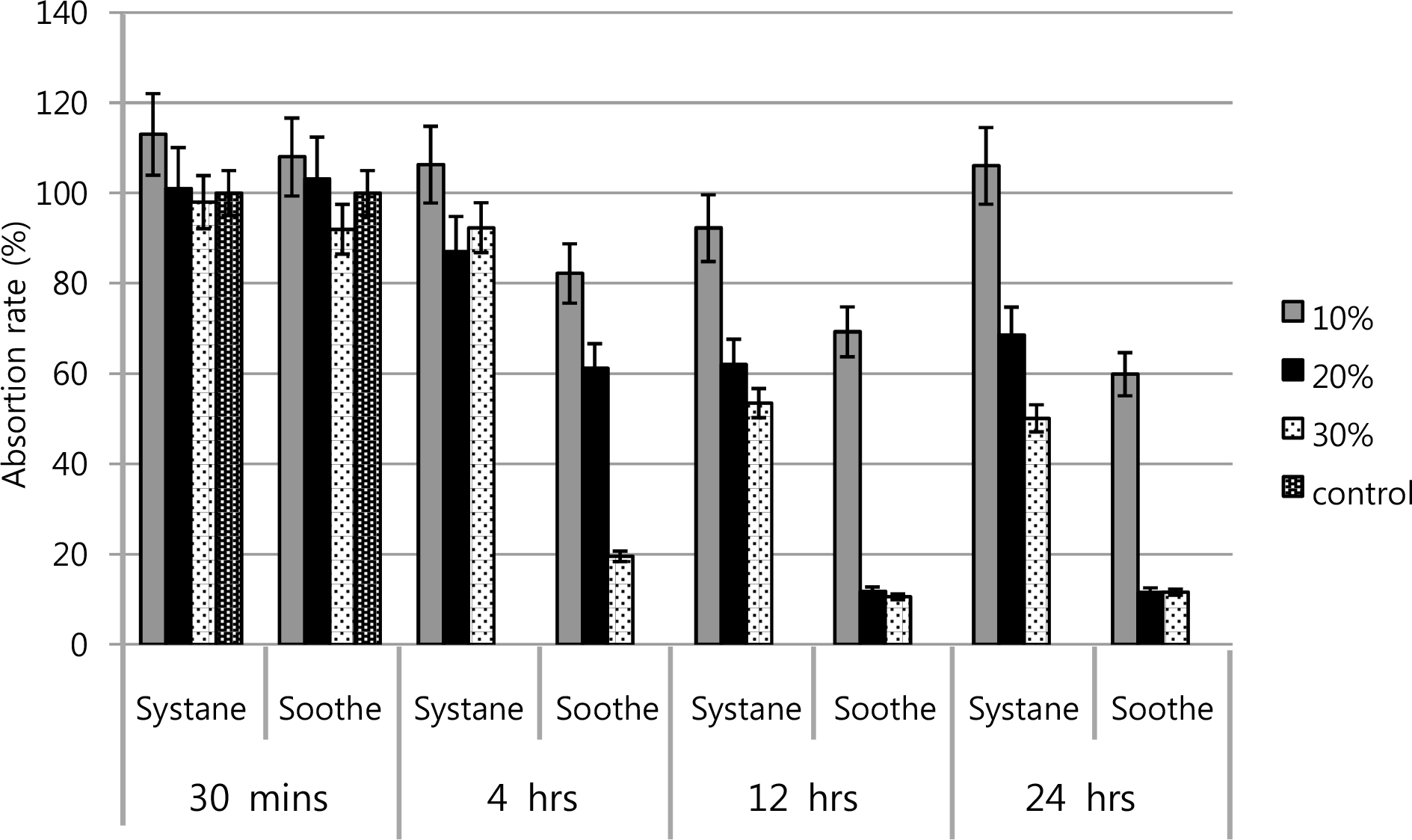
Figure 3.
LDH activities of cultured human corneal keratocytes showed that Systane® didn't affect LDH activity significantly, but Soothe® has showed that by dose and time-de-pendent response relationship.
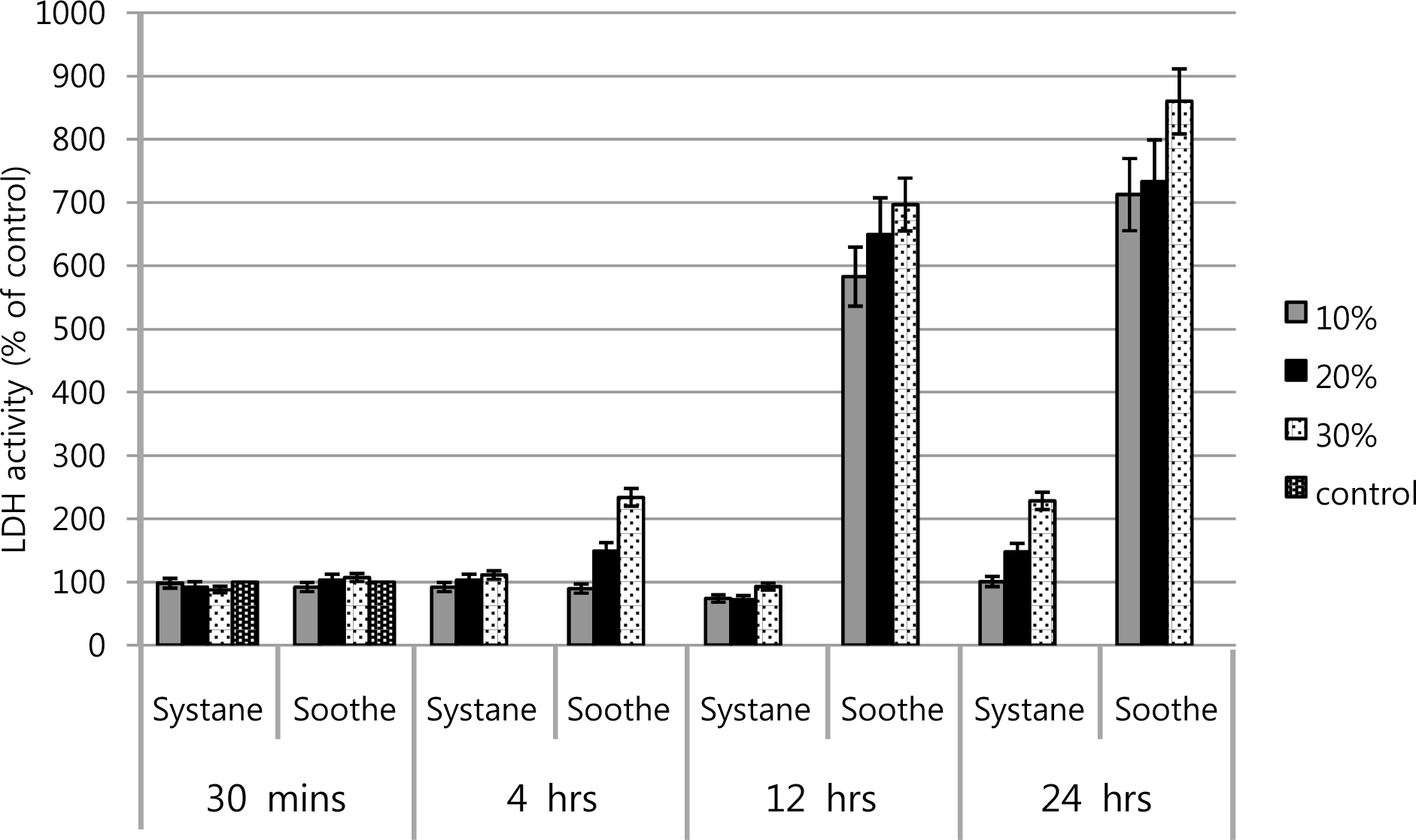
Figure 4.
LDH activities of cultured human conjunctival epithelial cells in Soothe® and Systane®. Soothe® showed greater LDH titer of the conjunctival cells than that of Systane®, especially in the rate of dilution of 20 and 30% after 12 hours exposure.
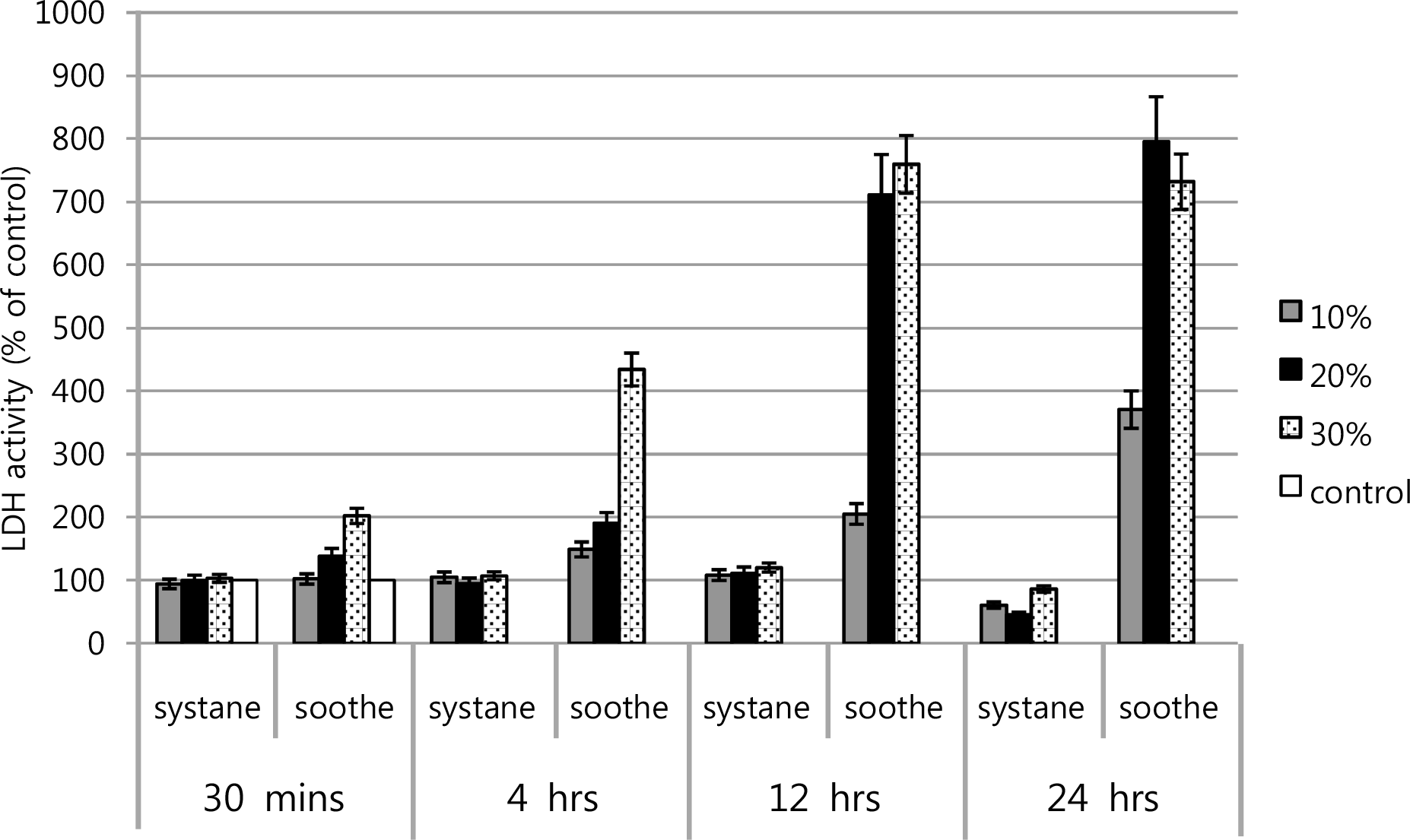
Figure 5.
Flow cytometric analysis of apoptotic cells using Annexin V-FITC at 30 minutes after exposure. Human corneal keratocytes were left untreated (A) or were treated with 10% (B), 20% (C), and 30% (D) by Soothe®, were treated with 10% (E), 20% (F), and 30% (G) by Systane®. After 30 minutes exposure, most corneal keratocytes in both artificial tears were primarily Annexin V and PI negative.
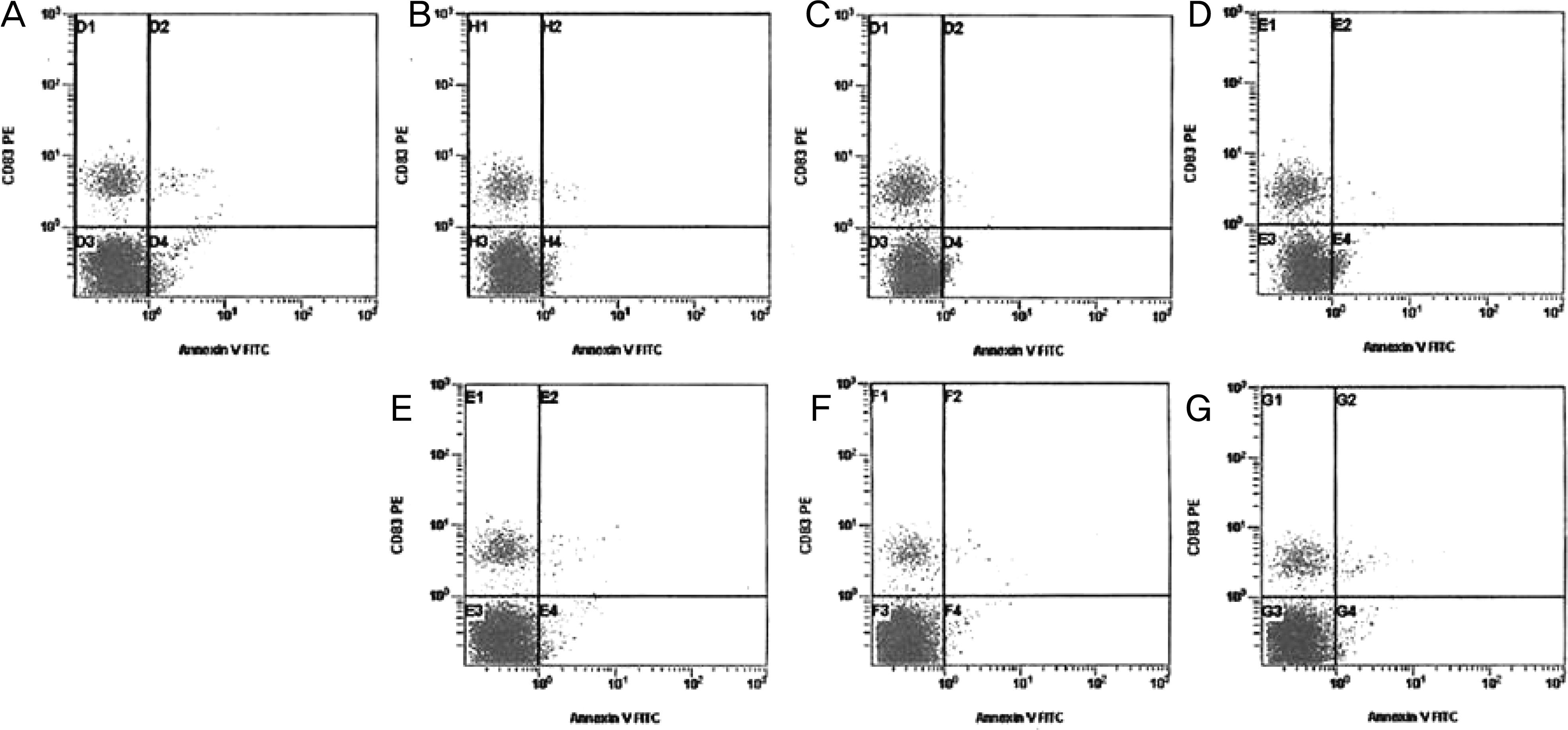
Figure 6.
Flow cytometric analysis of apoptotic cells using Annexin V-FITC at 4 hours after exposure. Human corneal keratocytes were left untreated (A) or were treated with 10% (B), 20% (C), and 30% (D) by Soothe®, were treated with 10% (E), 20% (F), and 30% (G) by Systane®. After 20% Soothe®, 30% Soothe® and 30% Systane® exposure, a number of cells are positive Annexin V and negative PI (C, D, G) indicating that the cells were in the stage of apoptosis.
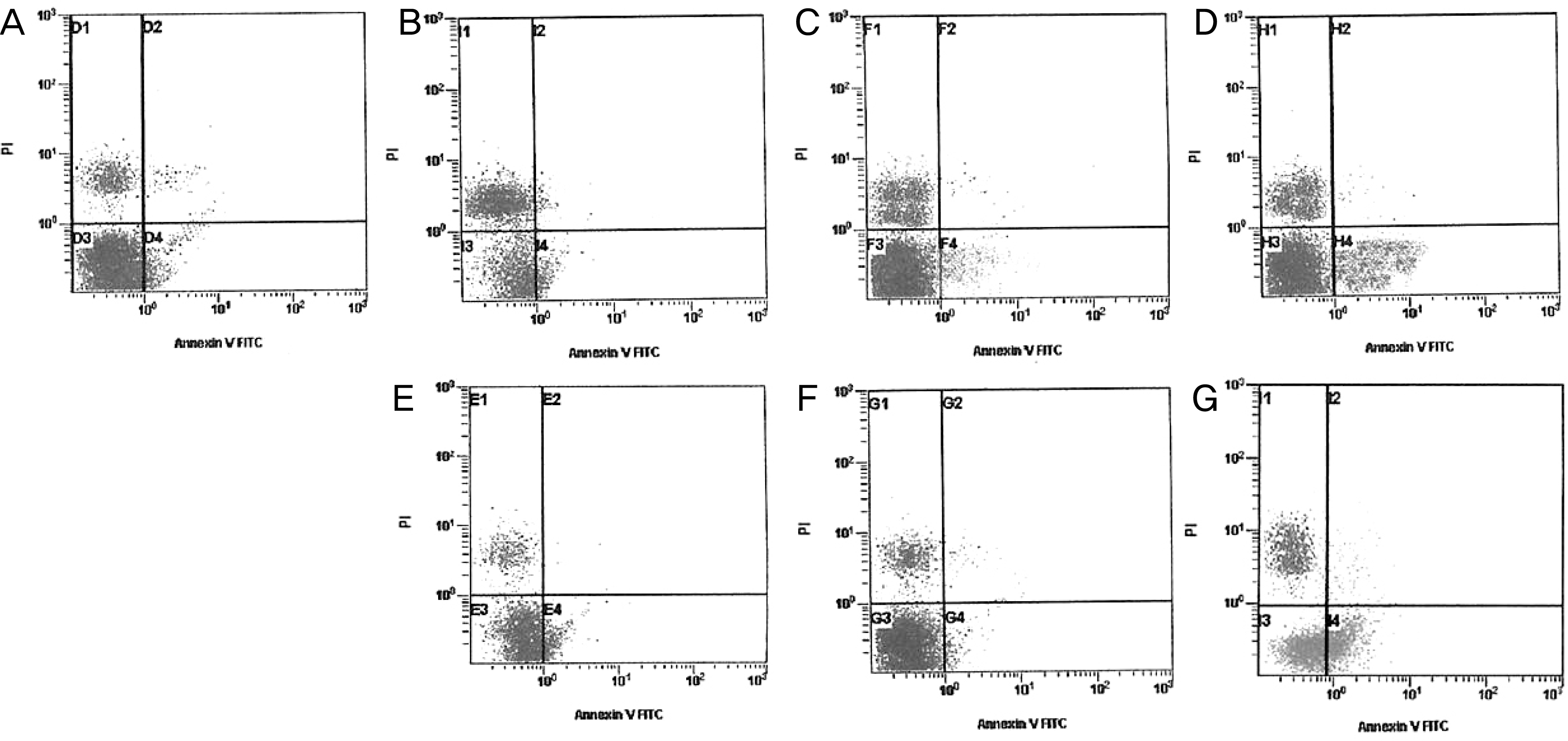
Figure 7.
Fluorescent microscopic findings of human conjunctival epithelial cells (A, B, C) and corneal keratocytes (D, E, F) at 4 hours after exposure (original magnification, ×200). (A, D) control. (B, E) 10% Soothe®. (C, F) 30% Systane®. After treating with Soothe® and Systane®, apoptotic cells were visible, which represent fluorescence after binding DNA flowing into cytoplasm due to disruption of nuclear membrane.
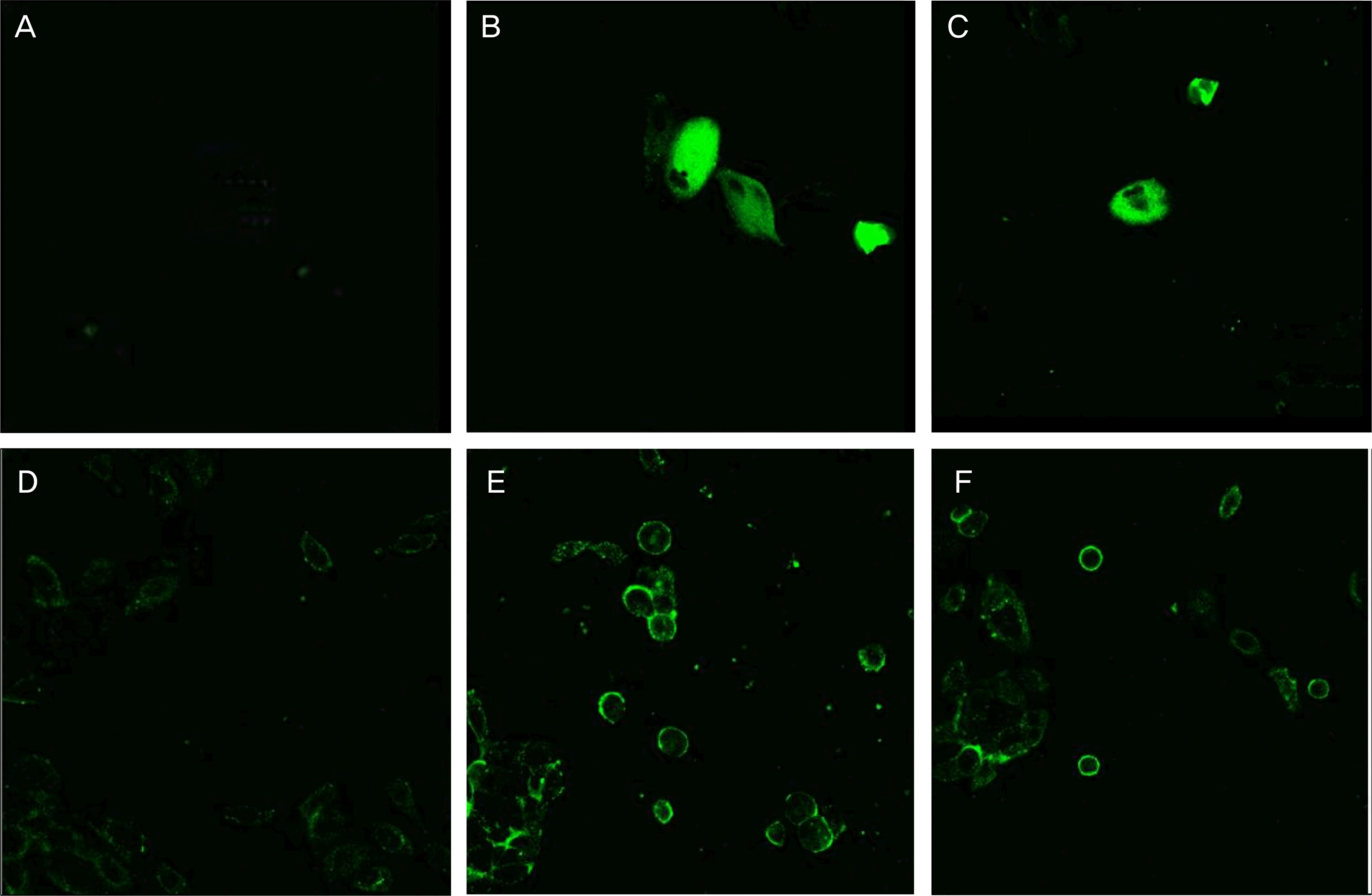
Figure 8.
Transmission electron micrograph of human corneal keratocytes (A, B, C) and human conjunctival epithelial cell (D, E, F) appeared after 4 hours exposure to (A, D) control, (B, E) 20% Soothe®, (C, F) 30% Systane®. (Bar length 2 μ m, original magnification, ×3,000∼4,000). In general, the plasma membrane with microvilli (black arrow), nuclear membrane, and nuclei of corneal keratocytes were visible. 20% Soothe® had more severe and damaged cellular structures, such as the plasma membranes with microvilli being disrupted (white arrow), vacuoles enlarge (black arrow head). The chromatin of the nuclear remnant had condensed along the nuclear periphery (white arrow head). The keratocyte and conjunctival epithelial cell showed loss of microvilli, vacuoles formation suspected swollen cytoplasmic organelles at the cytoplasm (black arrow head) compared with control after 4 hours exposure to 30% Systane®.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download