Abstract
Purpose
To investigate the outcomes of the combined therapy of intravitreal bevacizumab (IVB) and posterior sutbtenon triam-conolone acetonide (STTA) injections as compared to single injections of each in patients with diabetic macular edema (DME).
Methods
IVB injection (IVB group), STTA injection (STTA group) and combined therapy injection (combined group) were performed randomly for 90 eyes (83 patients) diagnosed with DME. Changes in central macular thickness (CMT), best corrected visual acuity (BCVA) and intraocular pressure (IOP) were compared among groups prospectively at pre-injection and one, three and six months after injection.
Results
Reduction of CMT and improvement of BCVA were maintained for three months after treatment in all groups, but CMT and BCVA deteriorated at six months after treatment. The combined therapy group revealed significant reductions in CMT and improvement of BCVA (p=0.003, p=0.021, respectively) for the first month compared to the single treatment group. Elevated IOPs were found in three cases and there was no endophthalmitis or retinal detachment.
References
1. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103:1796–806.
2. Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report number 19. Arch Ophthalmol. 1995; 113:1144–55.
3. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.
4. Jonas JB. Intravitreal triamcinolone acetonide for diabetic retinopathy. Dev Ophthalmol. 2007; 39:96–110.

5. Spandau UH, Derse M, Schmitz-Valckenberg P, et al. Dosage de-pendency of intravitreal triamcinolone acetonide as treatment for diabetic macular edema. Br J Ophthalmol. 2005; 89:999–1003.
6. Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004; 122:336–40.
7. Cellini M, Pazzaglia A, Zamparini E, et al. Intravitreal vs. subtenon triamcinolone acetonide for the treatment of diabetic cystoid macular edema. BMC Ophthalmol. 2008; 8:5.

8. Kwon SJ, Shin JP, Kim SY. Intravitreal versus subtenon injections of triamcinolone acetonide for diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:81–90.

9. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

10. Funatsu H, Yamashita H, Ikeda T, et al. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002; 133:537–43.

11. Seo JW, Park IW. Intravitreal bevacizumab for treatment of diabetic macular edema. Korean J Ophthalmol. 2009; 23:17–22.

12. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6- month follow-up. Ophthalmology. 2007; 114:743–50.
13. Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006; 113:1706–12.

14. Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008; 145:854–61.

15. Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). Br J Ophthalmol. 2008; 92:76–80.

16. Kok H, Lau C, Maycock N, et al. Outcomes of intravitreal triamcinolone in uveitis. Ophthalmology. 2005; 112:1916–7.
17. Ruiz-Moreno JM, Montero JA, Zarbin MA. Photodynamic therapy and high-dose intravitreal triamcinolone to treat exudative age-related macular degeneration: 2-year outcomes. Retina. 2007; 27:458–61.
18. Lee SJ, Kim ES, Geroski DH, et al. Pharmacokinetics of intraocular drug delivery of Oregon Green 488-labeled triamcinolone by sub-tenon injection using ocular fluorophotometry in rabbit eyes. Invest Ophthalmol Vis Sci. 2008; 49:4506–14.

19. Ozkiris A, Erkilic K. Complications of intravitreal injection of triamcinolone acetonide. Can J Ophthalmol. 2005; 40:63–8.
20. Ciulla TA, Rosenfeld PJ. Anti-vascular endothelial growth factor therapy for neovascular ocular disease other than age-related macular degeneration. Curr Opin Ophthalmol. 2009; 20:166–74.
21. Soheilian M, Ramezani A, Bijanzadeh B, et al. Intravitreal bevacizumab (Avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. 2007; 27:1187–95.

Figure 1.
Changes in central macular thickness (CMT) after treatments in the three groups. Reduction of CMT was maintained at 3 months after injection in intravitreal bevacizumab (IVB) injection group, subtenon triamcinolone (STTA) injection group, and combined therapy group. CMT increased at 6 months after injection, but final CMT was still significantly lower than initial CMT. Combined therapy revealed significant reduction of CMT compared to other treatments at 1 month after treatment (* p=0.003). There was no difference among groups at 6 months after treatment († p=0.548).
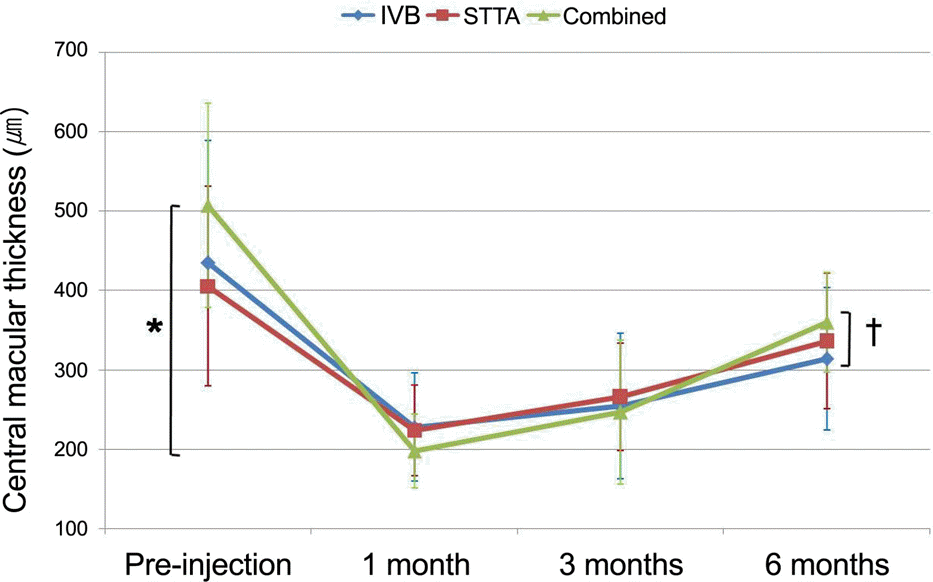
Figure 2.
Changes in visual acuity (Va, LogMAR) after treatments in the three groups. Improvement of CMT was maintained at 3 months after injection in intravitreal bevacizumab (IVB) injection group, subtenon triamcinolone (STTA) injection group, and combined therapy group. Va became worse at 6 months after injections, but final Va was still significantly better than initial Va. Combined therapy revealed significant improvement of Va compared to other treatments at 1 month after treatment (* p= 0.021). There was no difference among groups at 6 months after treatment († p=0.139).
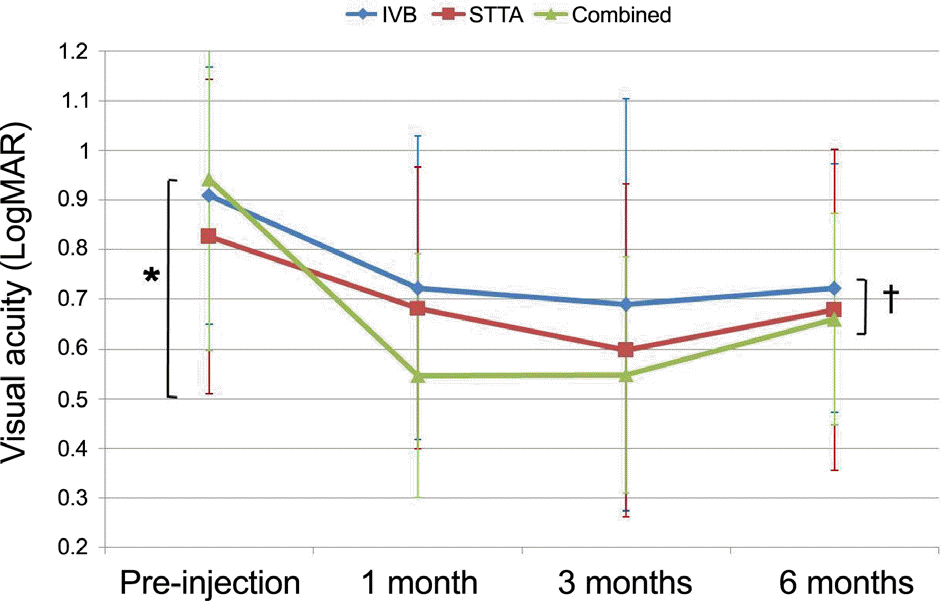
Figure 3.
Comparisons of changes in intraocular pressure (IOP) among groups at each period after treatments. There was no significant difference of changes in IOP among groups at each period.
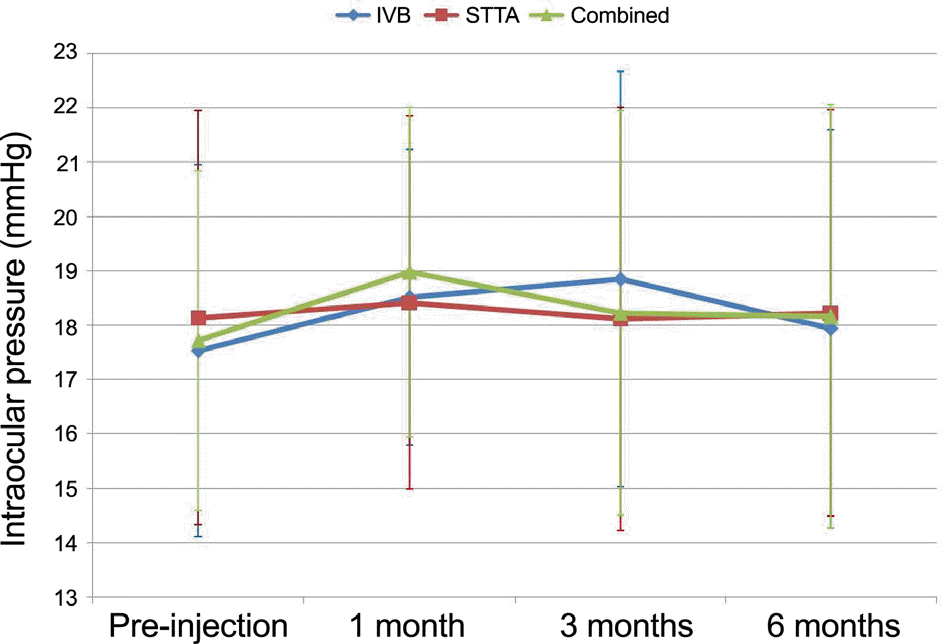
Table 1.
Characteristics of subjects in three groups
| IVB (30 eyes) | STTA (30 eyes) | Combined (30 eyes) | p-value | |
|---|---|---|---|---|
| Age (years) | 61.6±10.0 | 61.3±10.6 | 58.6±11.1 | 0.463* |
| Gender (M / F) | 16 / 14 | 18 / 12 | 11 / 19 | |
| Hypertension | 8 | 11 | 13 | 0.180† |
| HbA1c (mg/dL) | 6.84±1.03 | 7.54±1.22 | 7.78±1.78 | 0.283* |
| Initial Va‡ | 0.91±0.26 | 0.83±0.32 | 0.94±0.35 | 0.381* |
| Initial CMT§ (μm) | 434.5±154.3 | 425.5±125.8 | 502.9±126.4 | 0.116* |
| Initial IOP∏ (mmHg) | 17.53±5.42 | 18.44±4.81 | 17.72±4.12 | 0.257* |
| IOP∏↑ (≥30 mmHg) | 2 | 0 | 1 | 0.474† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download