Abstract
Purpose
To investigate the effect of methylglyoxal (MG), intermediate metabolite of advanced glycation end products(AGE), on the induction of oxidative stress in human trabecular meshwork cells (HTMC).
Methods
Primarily cultured HTMC were exposed to at concentrations of 0, 30, 100, and 300 μm of MG for 18 hours, with or without co-exposure to N-acetyl-cysteine. Cellular survival and apoptosis were assessed by MTT assay and flow cytometry using annexin-PI double staining. Production of nitric oxide (NO), superoxide, and reactive oxygen species (ROS) was assessed by Griess assay, cytochrome c assay, and dichlorofluorescein diacetate assay, respectively.
References
1. Wiederholt M. Dirtect involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr Opin Ophthalmol. 1998; 9:46–9.
2. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.

3. Rohen JW, LÜtjen-drecoll E, FlÜgel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma. Exp Eye Res. 1993; 56:683–92.
4. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.
5. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.

6. Brüne B, Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.

7. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.
8. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eyes. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.
9. Meyer P, Champion C, Schlotzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.

10. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and ocular facility in monkeys. Exp Eye Res. 1994; 58:99–105.
11. Wana RF, Podos SM. Effect of the topical application of nitro-glycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.
12. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.
13. Matsuo T. Basic nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.
15. Kurz DJ, Decary S, Hong Y, et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004; 117:2417–26.

16. Vasa M, Breitchopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerases and delays endothelial cell senescence. Circ Res. 2000; 540–2.
17. Bucala R, Vlassara H, Cerami A. Advanced glycation end products. Vol. 2. Boca Raton: CRS Press;1992. p. 53–9.
18. Bucala R, Tracey KJ, Cerami A. Advanced glycation end products quench nitric oxide and mediate defective endothelium-dependent vasodilatationin experimental diabetes. J Clin Invest. 1991; 87:432–8.
19. Franke S, Dawczynski J, Strobel J, et al. Increase levels of advanced glycation end products in human cataractous lenses. J Cataract Refrac Surg. 2003; 29:998–1004.
20. Nakamura N, Hasegawa G, Obayashi H, et al. Increased concentration of pentosidine, an advanced glycation end product, and interleukin-6 in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2003; 61:93–101.

21. Yim HS, Kang SO, Hah YC, et al. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. J Biol Chem. 1995; 270:28228–33.

22. Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002; 39:809–14.

23. Kang JH. Oxidative damage of DNA induced by methylglyoxal in vitro. Toxicol Lett. 2003; 145:181–7.

24. Chang T, Wang R, Wu L. Methylglyoxal-induced nitric oxide and peroxynitrite production in vascular smooth muscle cells. Free Radi Biol Med. 2005; 38:286–93.

25. Valencia JV, Weldon SC, Quinn D, et al. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem. 2004; 324:68–78.

26. Shamsi FA, Lin K, Sady C, Nagaraj RH. Methylglyoxal-derived modifications in lens aging and cataract formation. Invet Ophthalmol Vis Sci. 1998; 39:2355–64.
27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

28. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15 N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.
29. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods. 1995; 184:39–51.
30. Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44:276–87.
31. Teufelhofer O, Weiss R-M, Parzefall W, et al. Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular superoxide. Toxicolol Sci. 2003; 76:376–93.

32. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–16.
33. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecularcells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.
34. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.
35. Schimidt AM, Hori O, Brett J, et al. Cellular receptor for advanced glycation end products: implications for induction of oxidative stress and cellular dysfunction in the pathogenesis of vascular lesions. Atheroscler Thromb. 1994; 14:1521–28.
36. Ramasamy R, Vannucci S, Yan SSD, et al. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiol. 2005; 15:16–28.

37. Stitt AW, Moore JE, Sharkey JA, et al. Advanced glycation end products in vitreous: structural and functional implications for diabetic vitreopathy. Invest Ophthalmol Vis Sci. 1998; 39:2517–23.
38. Stitt AW. Advanced glycation: An important pathological event in diabetic and age related ocular disease. Br J Ophthamol. 2001; 85:746–53.

38. Stitt AW. Role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003; 57:95–108.
40. Kaji Y, Usui T, Oshika T, et al. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci. 2000; 41:362–8.
41. Pokupec R, Kalauz M, Turk N, Turk Z. Advanced glycation end products in human diabetic and non-diabetic cataractous lenses. Graefes Arch Clin Exp Ophthalmol. 2003; 241:378–4.
42. Amano S, Kaji Y, Oshika T, et al. Advanced glycation end products in human optic nerve head. Br J Ophthalmol. 2001; 85:52–5.

43. Alvarado JA, Alvarado RG, Yeh RF, et al. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm's canal endothelial cells. Br J Ophthalmol. 2005; 89:1500–5.

44. Madamanchi NR, Vendov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vas Biol. 2005; 25:29–38.

45. Chakravarthy U, Hayes RG, Stitt RW, et al. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998; 47:945–52.

46. Uhlmann S, Rezzoug K, Friedrichs U, et al. Advanced glycation end products quench nitric oxide in vitro. Graefes Arch Clin Exp Ophthalmol. 2002; 240:860–6.

47. Sagga S. Nitric oxide as a mediator of glaucoma pathogenesis. Med Sci Monit. 2002; 8:33–4.
49. Schachtschabel DO, Binninger EA, Rohen JW. In vitro cultures of trabecular meshwork cells of the human eye as a model system for the study of cellular aging. Arch Gerontol Geriat. 1989; 9:251–62.

50. Ferreira SM, Lerner SF, Brunzini R, et al. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004; 137:62–9.

51. Yildirim O, Ates NA, Ercan B, et al. A. Role of oxidativestress enzymes in open-angle glaucoma. Eye. 2005; 19:580–3.
52. Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003; 114:638–46.

53. Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in human trabecular meshwork. Arch Ophthalmol. 2005; 123:458–63.
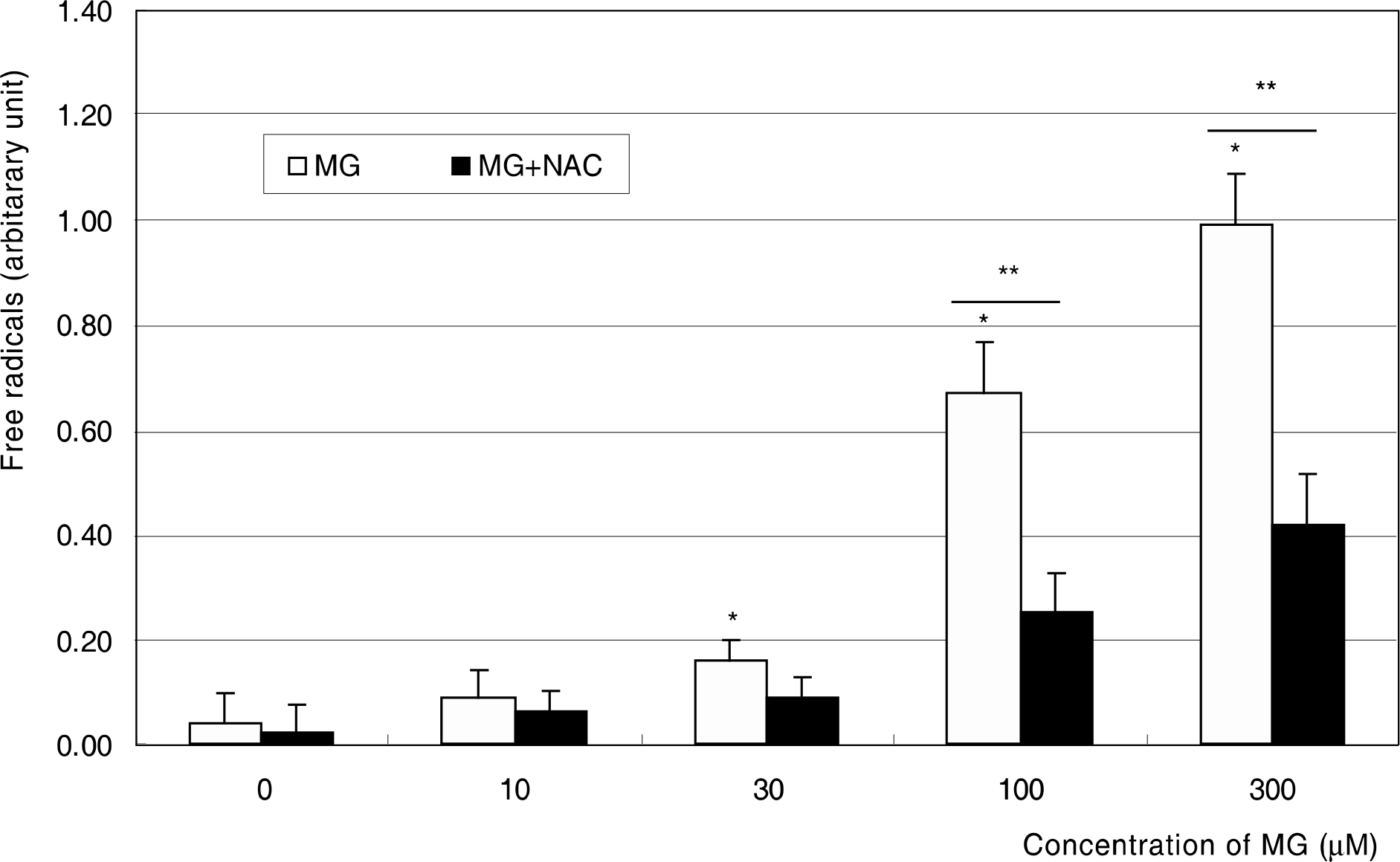
Figure 1.
Effect of methylglyoxal (MG) on the survival of trabecular meshwork cells. MG and co-exposure of 50 μM N-acetyl-cysteine (NAC) did not affect on the cellular survival significantly except at 300 μM MG. (* p<0.05)
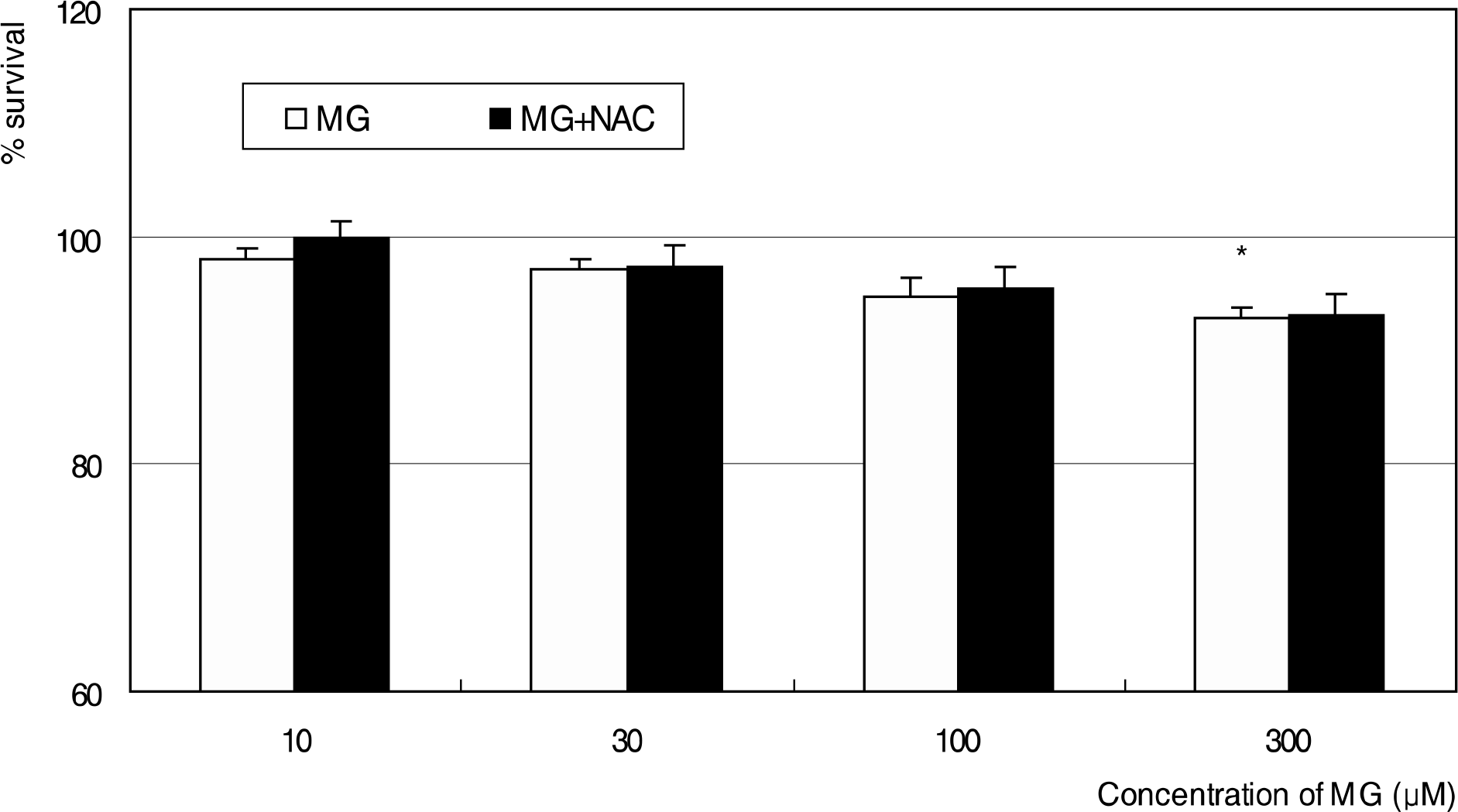
Figure 2.
Effect of methylglyoxal on the induction of apoptosis in cultured trabecular meshwork cells. Methylglyoxal induced apoptosis from 100 μM. (* p<0.05)
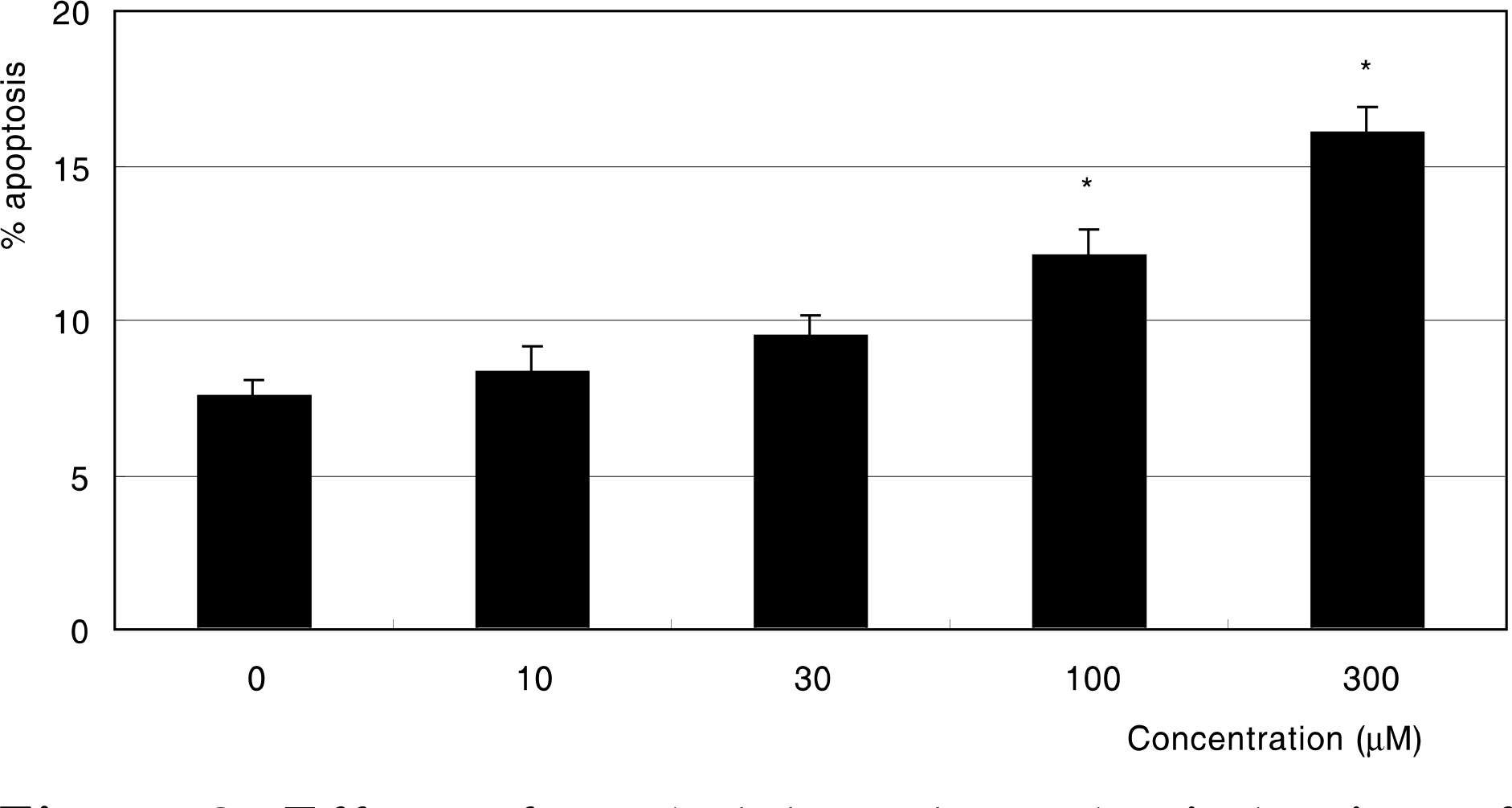
Figure 3.
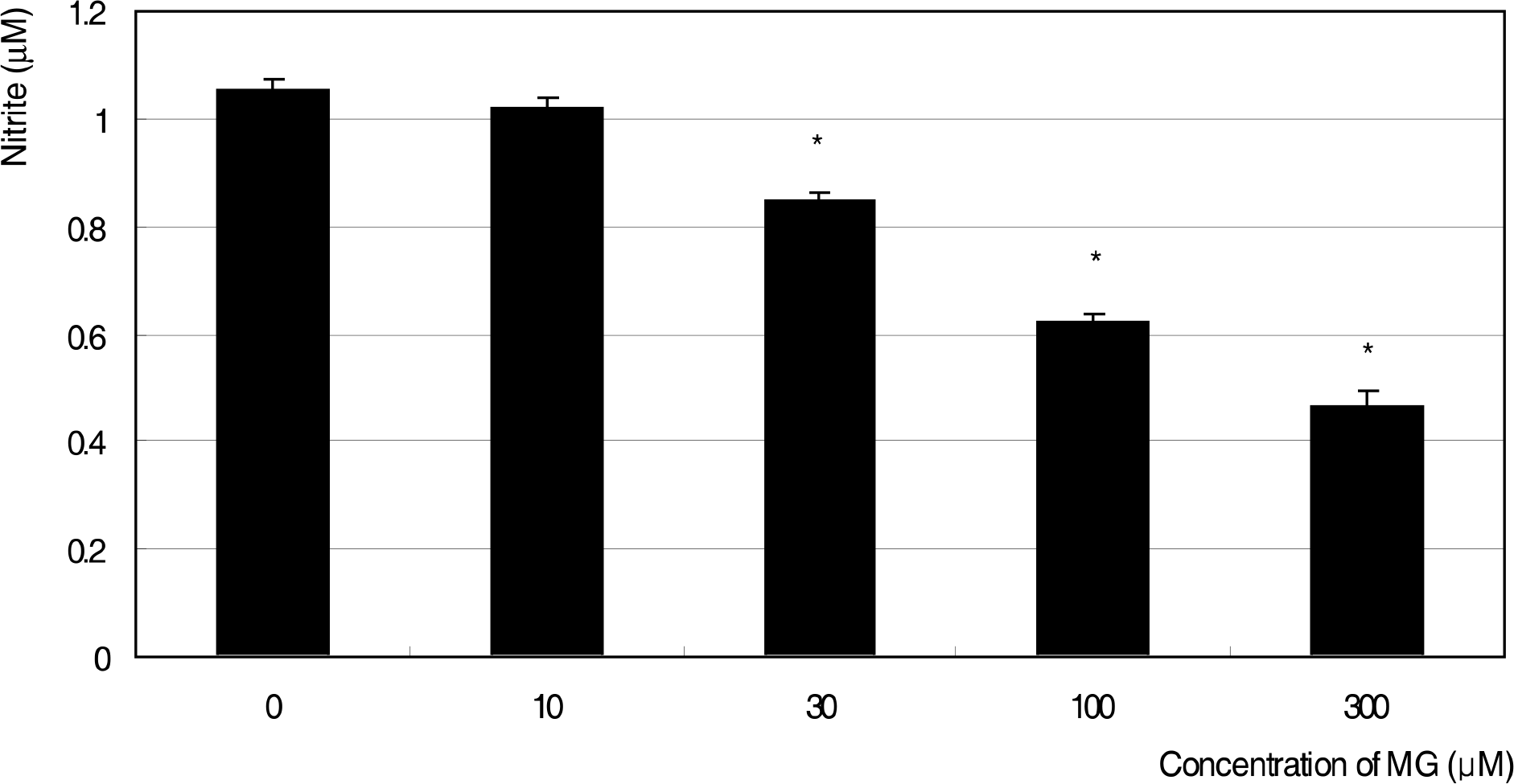
Effect of methylglyoxal (MG) on the generation of nitric oxide in cultured trabecular meshwork cells. MG decreased nitric oxide production from 30 μM. (* p<0.05)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download