Abstract
Methods
The electroretinogram (ERG) waves were recorded in 52 eyes of 26 normal subjects with UTAS E-3000® (LKC Technologies Inc., Gaithersberg, MD, USA). Photopic cone response was obtained for analysis from a white flash with white background illumination (group 1) and a red flash with blue background illumination (group 2), after stimulations ranging from 0.007674 cd.s/m2 (-25dB) to 7.736 cd.s/m2 (5dB) with a 5dB interval.
Results
PhNRs were observed in all 52 eyes in group 1 stimulated with the white flash at 0.9933 cd.s/m2 (-4dB). PhNRs were also observed in all 52 eyes in group 2 at 2.4297 cd.s/m2 (0dB) after stimulation with the red flash. There was correlation between the amplitudes of PhNR and intensity of stimuli (p <0.001). Implicit times of PhNR were correlated with age in both groups, but amplitudes decreased with age in group 1.
References
1. Marmer MF, Arden GB, Nisson SE, Zrenner E. Standard for clinical eletroretinography. Arch Ophthalmol. 1989; 107:816–9.
2. Marmer MF, Zrenner E. Standard for clinical electroretinography (1994 update). Doc Ophthalmol. 1995; 89:199–210.

3. Mammer MF. An updated standard for clinical electroretinography. Arch ophthalmol. 1995; 113:1375–6.

5. Bush RA, Sieving PA. A proxima retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci. 1994; 35:635–45.
6. Sieving PA, Murayama K, Naarendorp F. Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci. 1994; 11:519–32.

7. Drasdo N, Aldedasi YH, Chiti Z, et al. The s-cone PhNR and pattern ERG in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001; 42:1266–72.
8. Viswanathan S, Frishman LJ, Robson JG, Walters JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001; 42:514–22.
9. Colotto A, Falsini B, Salgarello T, et al. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000; 41:2205–11.
10. Machida S, Gotoh Y, Tanaka M, Tazawa Y. Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol. 2004; 137:938–40.

11. Peyman GA, Sanders DR, Galdberg NR. Principles and practice of ophthalmology. Philadelphia: WB Saunders Co.;1980. p. 857.
12. Riggs LA. Continuous and reproducible records of electrical activity of the human retina. Proc Soc Exp Biol Med. 1941; 48:204–7.
13. Viswanathan S, Frishman LJ, Robson JG, et al. The photopic negative response of the macaque electroretingram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999; 40:1124–36.
14. Drasdo N, Aldebasi YH, Mortlock KE, et al. Ocular optics, electroretinography and primary open angle glaucoma. Ophthalmic Physiol Opt. 2002; 22:455–62.

15. Miyata K, Nakamura M, Kondo M, et al. Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci. 2007; 48:820–4.
16. Chen H, Wu D, Huang S, Yan H. The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol. 2006; 113:53–9.

17. Cursiefen C, Korth M, Horn FK. The negative response of the flash electroretinogram in glaucoma. Doc Ophthalmol. 2001; 103:1–12.
18. Aguilar M, Stiles WS. Saturation of the rod mechanism of the retina at the high levels of stimulation. Optica Acta. 1954; 1:59–65.
19. Danias J, Lee KC, Zamora MF, et al. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/1NNia glaucomatous mice: Comparison with RGC loss in aging C57/BL6 mice. Invest Ophthlmol Vis Sci. 2003; 44:5151–62.
Figure 1.
Electroretinogram (ERG) intensity series to white stimuli on white background (30 cd/m2) and red stimuli on blue background (20 cd/m2). Photopic negative response (PhNR) was elicited on all of 52 eyes at 0.9933 cd.s/m2(-4dB) with white stimuli. PhNR was elicited on all of 52 eyes at 2.4297 cd.s/m2 (0dB) with red stimuli.
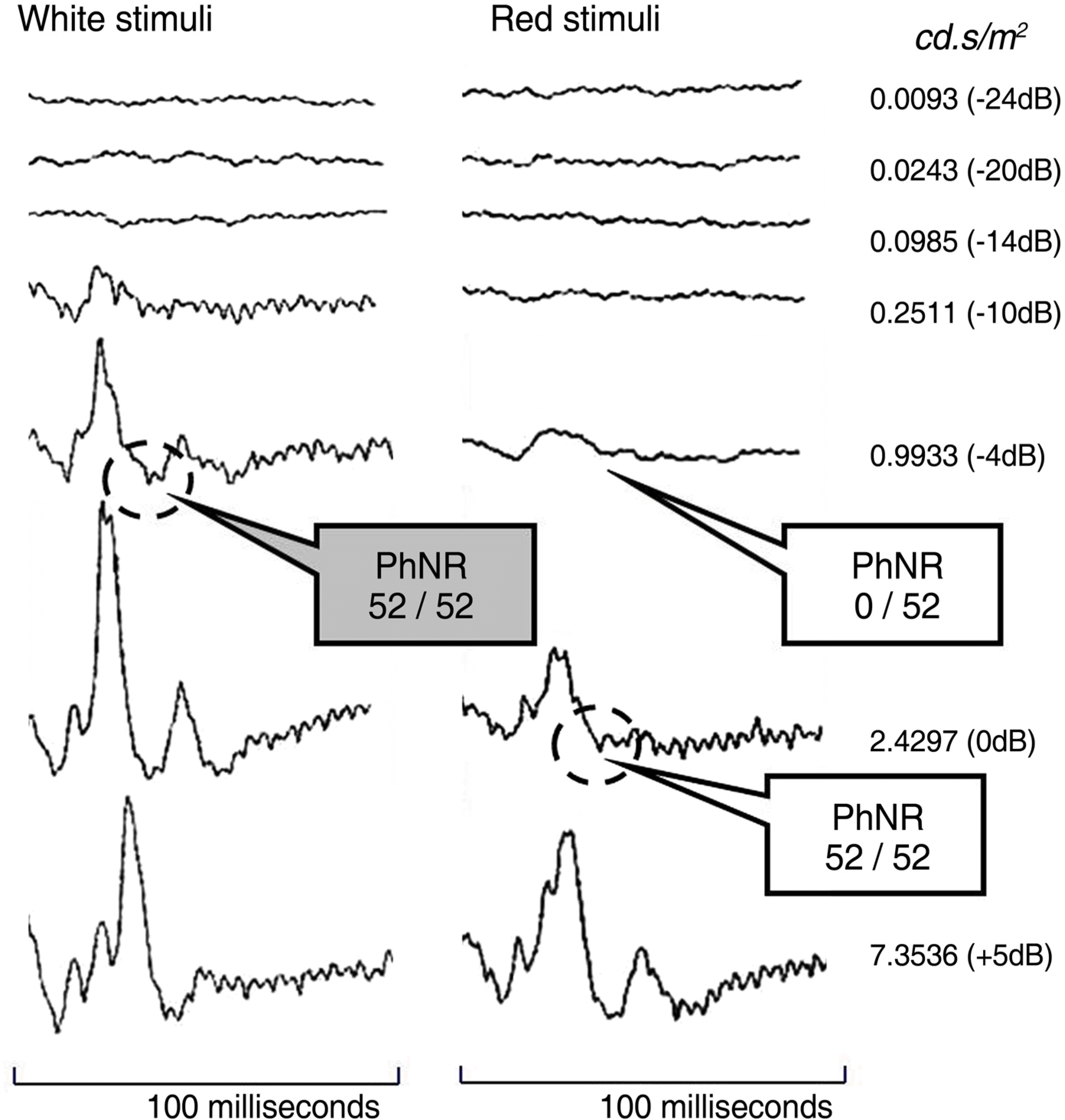
Figure 2.
Amplitudes of PhNR were correlated with intensity of stimuli in group 1 (A, p<0.001) and group 2 (B, p<0.001). But there was no correlation between implicit times of PhNR and intensity of stimuli in group 1 (C, p=0.482) and group 2 (D, p=0.076).
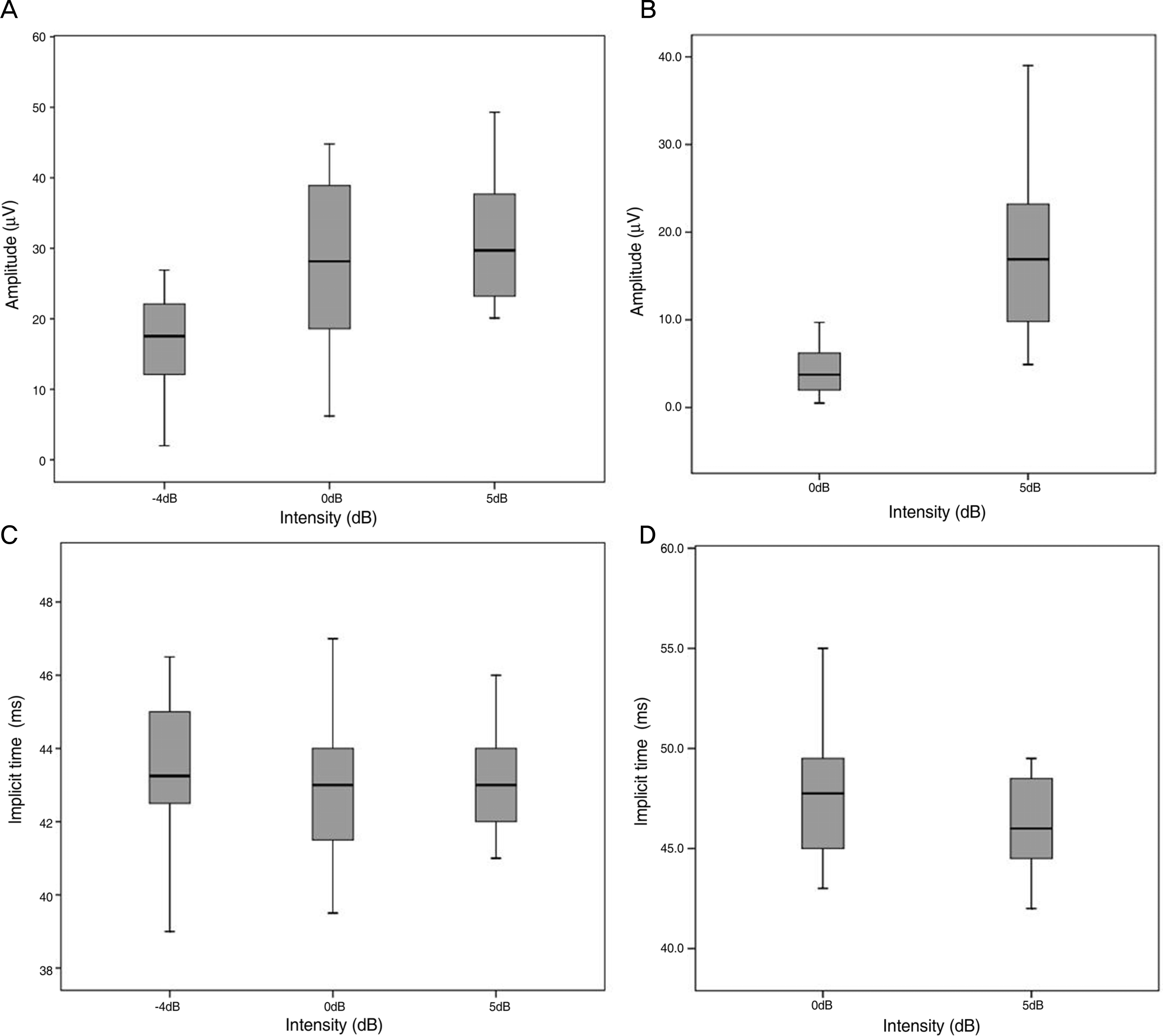
Figure 3.
Amplitudes of PhNR were decreased with age in group 1 (A, p<0.001). Implicit times of PhNR were increased with age (B, p=0.004).
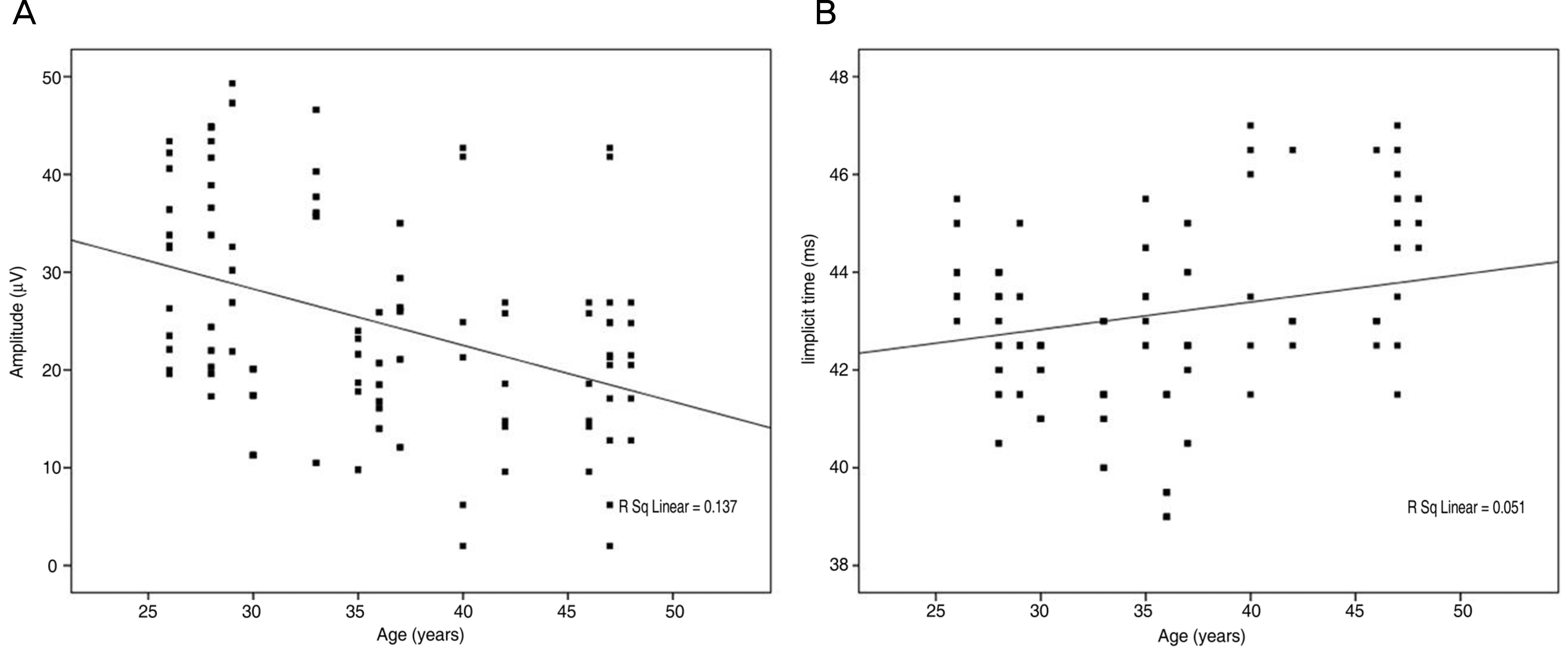
Figure 4.
Amplitudes of PhNR were not significantly changed according to age in group 2 (A, p=0.232). But implicit times of PhNR were significantly increased with age (B, p<0.001).
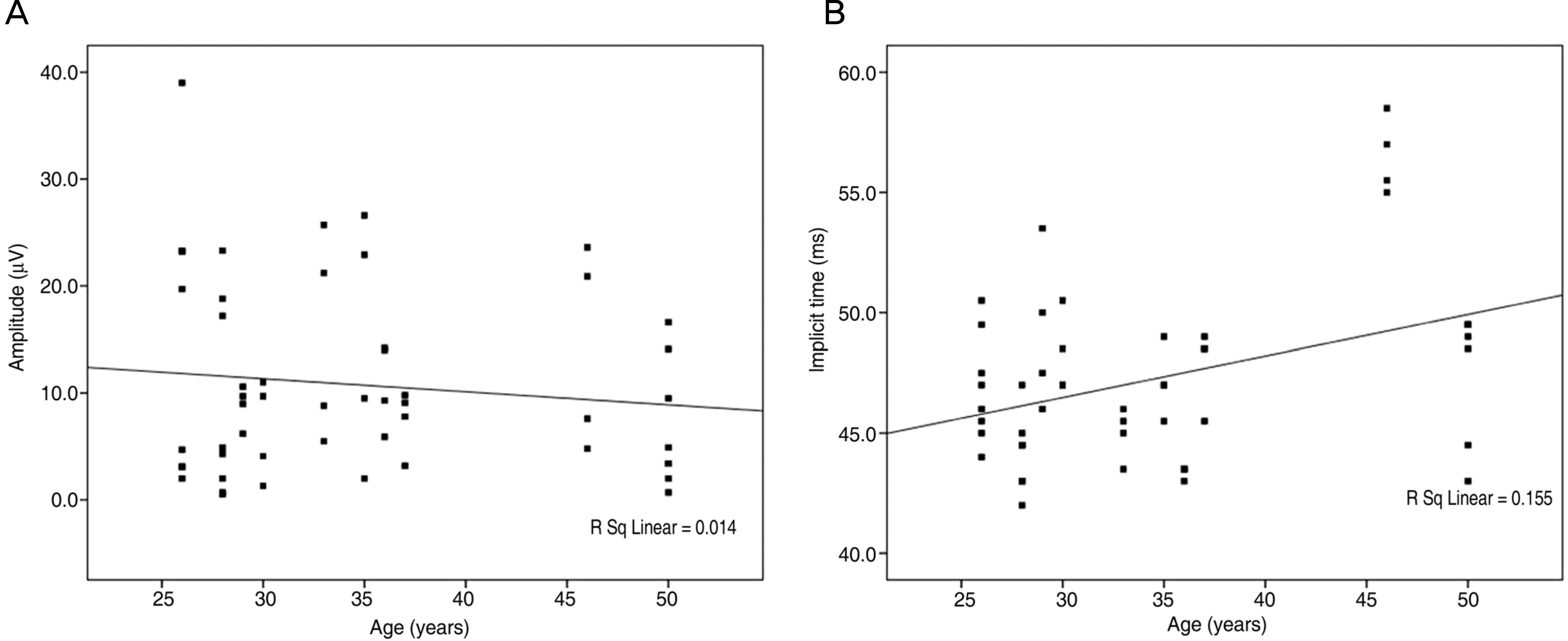
Table 1.
Mean amplitudes and implicit times of PhNR in group 1 (white stimulus on white background) and group 2 (red stimulus on blue background) according to the intensity of stimulation
| Intensity of stimulation | 0.9933 cd.s/m2 (-4dB) | 2.4297 cd.s/m2 (0dB) | 7.3536 cd.s/m2 (5dB) | p-value |
|---|---|---|---|---|
| Group 1 (White stimulus) | ||||
| Amplitude (uV) | 18.18±8.32 | 28.29±10.87 | 31.34±9.15 | <0.001* |
| Implicit time (ms) | 43.23±1.94 | 42.83±1.80 | 43.12±1.49 | 0.482* |
| Group 2 (Red stimulus) | ||||
| Amplitude (uV) | 4.50±2.93 | 16.98±7.91 | <0.001† | |
| Implicit time (ms) | 47.96±3.75 | 46.69±3.47 | 0.076† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download