Abstract
Purpose
We conducted a study to evaluate the change of tear secretion and symptoms in the patients with dry eye syndrome after using Restasis® (Cyclosporine 0.05% ophthalmic emulsion, Allergan Inc., U.S.A.).
Methods
We randomly selected 39 patients from newly or previously diagnosed dry eye syndrome patients and administered Restasis® to them. We checked their clinical parameters and symptoms over a period of 3 months. The clinical parameters evaluated were type I and type II Schirmer tests and tear break-up time, and the symptoms of dry eye syndrome were classified into pain, itching, foreign body sensation, blurred vision, and photophobia using a scoring scale for symptoms of 0 to 5. The results were analyzed with a Mann-Whitney test ( P-values <0.05 was considered statistically significant).
Results
For 26 of 39 patients (52 eyes) on whom all tests were carried out for 3 months, there was a significant improvement after 3 months in the type I Schirmer test, type II Schirmer test, and tear break-up time ( P=0.012, 0.009, 0.001, respectively). Only 14 patients completed the questionnaire for scoring of symptoms. After using Restasis®, foreign body sensation only improved ( P=0.010).
Conclusions
In our study, tear secretion was increased by Restasis®, and a greater increase in tear secretion was seen in patients with systemic disease than in patients without systemic disease. Additional patients need to be evaluated and longer-term studies need to be performed to confirm our results.
References
1. Pflugfelder SC, Tseng SC, Sanabria O. . Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998; 17:38–56.

2. Darsun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal epithelial erosions with inhibitors of matrix metalloproteinases-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001; 132:8–13.
3. Marsh P, Pflugfelder SC. Topical nonpreserved methylpredni solone-plus therapy of keratoconjunctivitis sicca in Sjogren's syndrome. Ophthalmology. 1999; 106:509–12.
4. Gündüz K, Ozdemir O. Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjogren's syndrome. Acta Ophthalmol. 1994; 72:438–42.
5. Tsubota K, Goto E, Fujita H. . Treatment of dry eye by autologous serum application in Sjogren's syndrome. Br J Ophthalmol. 1999; 83:390–5.

6. Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000; 19:644–9.
7. Stern ME, Beuerman RW, Fox RI. . The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–9.
8. Stern ME, Gao J, Siemasko KF. . The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78:409–16.

9. Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001; 85:147–53.

10. Solomon A, Dursun D, Liu Z. . Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001; 42:2283–92.
11. Balaram M. . Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2002; 43:1004–11.
12. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002; 120:330–7.

13. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000; 107:631–9.
14. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporine A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Cyclosporine A Phase 2 Study Group. Ophthalmology. 2000; 107:967–74.
15. Brignole F, Pisella PJ. . Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporine A. Invest Ophthalmol Vis Sci. 2001; 42:90–5.
16. Kunert KS, Tisdale AS, Stern ME. . Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000; 118:1489–96.
Figure 1.
Schirmer test and tear breakup time changes (Δ) from baseline measured at 1 month, 2 months, 3 months, respectively (n=52).
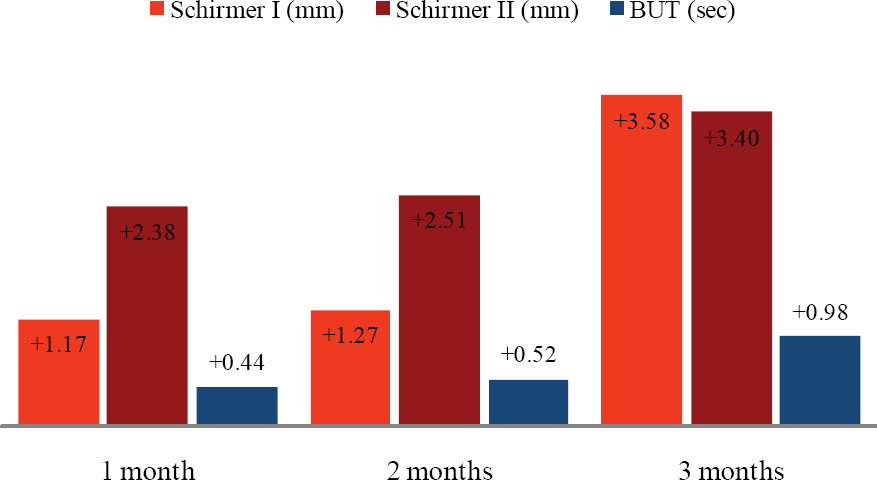
Figure 2.
Comparison of increase amount in Schirmer Test with or, without systemic disease (n : number of eyes).
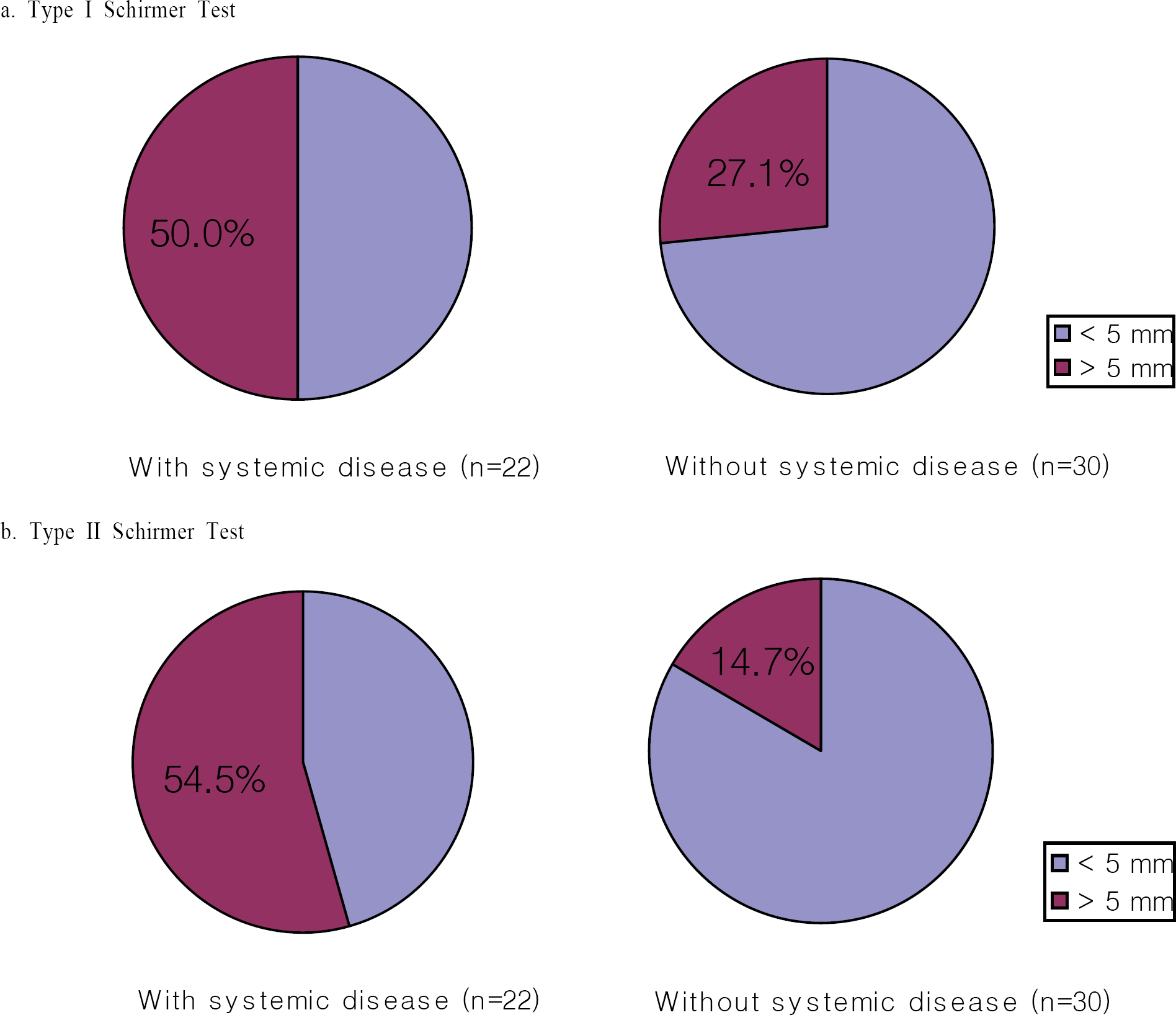
Table 1.
Characteristics of study group at screening
| Total number of group | n=39 |
| Average of age (years) | 45.67±14.19 |
| Gender | Male:14 Female:25 |
| History of ocular surgery | 6 |
| Systemic disease* | 15 |
| No. of patients using other artificial tear drops | 28 |
Table 2.
Schirmer test and tear breakup time during 3 months (n=52, Mann-Whitney test)
| First visit | After 1 month | After 2 months | After 3 months | ||
|---|---|---|---|---|---|
| Schirmer Test 1 (mm) | Average | 10.00±7.23 | 10.35±5.44 | 10.44±6.12 | 12.67±7.11 |
| P value | 0.531 | 0.776 | 0.012 | ||
| Schirmer Test 2 (mm) | Average | 7.00±4.88 | 8.60±4.68 | 8.10±4.55 | 9.48±5.30 |
| P value | 0.103 | 0.284 | 0.009 | ||
| BUT* (sec) | Average | 6.30±2.31 | 6.75±2.12 | 6.83±1.98 | 7.29±1.95 |
| P value | 0.227 | 0.055 | 0.001 |
Table 3.
Symptom score of dry eye syndrome during 3 months (number of patient participating in Questionnaire=14, Mann-Whitney test)
| Symptom Score* | First visit | After 1 month | After 2 months | After 3 months | |
|---|---|---|---|---|---|
| Irritation | average | 3.07 | 3.14 | 2.57 | 2.57 |
| P value | 0.739 | 0.121 | 0.107 | ||
| Itching | average | 1.78 | 1.43 | 1.43 | 1.43 |
| P value | 0.102 | 0.336 | 0.380 | ||
| Foreign body sensation | average | 3.00 | 2.26 | 2.20 | 2.16 |
| P value | 0.024 | 0.028 | 0.010 | ||
| Blurred vision | average | 2.21 | 1.71 | 1.71 | 1.93 |
| P value | 0.084 | 0.154 | 0.365 | ||
| Photophobia | average | 2.79 | 2.43 | 3.14 | 2.86 |
| P value | 0.129 | 0.059 | 0.796 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download