Abstract
Objective
Low back pain, caused intervertebral disc degeneration has been treated by thermal annuloplasty procedure, which is a non-surgical treatement. The theoretical backgrounds of the annuloplasty are thermal destruct of nociceptor and denaturization of collagen fiber to induce contraction, to shrink annulus and thus enhancing stability. This study is about temperature and its distribution during thermal annuloplasty using 1414 nm Nd : YAG laser.
Methods
Thermal annuloplasty was performed on fresh human cadaveric lumbar spine with 20 intact intervertebral discs in a 37℃ circulating water bath using newly developed 1414 nm Nd : YAG laser. Five thermocouples were attached to different locations on the disc, and at the same time, temperature during annuloplasty was measured and analyzed.
Results
Thermal probe's temperature was higher in locations closer to laser fiber tip and on lateral locations, rather than the in depth locations. In accordance with the laser fiber tip and the depth, temperatures above 45.0℃ was measured in 3.0 mm depth which trigger nociceptive ablation in 16 levels (80%), in accordance with the laser fiber end tip and laterality, every measurement had above 45.0℃, and also was measured temperature over 60.0℃, which can trigger collagen denaturation at 16 levels (80%).
Low back pain (LBP) is thought to have originated from intervertebral lesion (7–39%). Most patients with discogenic LBP respond well to conservative care, but 5% of patients suffer from constant pain313). Various treatment modalities, such as injection therapy, fusion surgery, total disc replacement, and thermal annular procedure, are used for discogenic LBP, and yet clinical efficacy is controversial125678111925). Until now, there were some thermal therapies for discogenic LBP, namely, intradiscal electrothermal therapy (IDET) using thermocoil located at the nucleo-annular junction, discTRODE™ using monopolar radiofrequency to apply ionic heat, and biacuplasty using cooled bipolar radiofrequency81624). According to Lee et al.17), annuloplasty was performed using laser as a heat source and a 90% success rate was reported.
Heat usage in the treatment of discogenic LBP is based on two rationale. First, using heat causes denervation of the nociceptor in the annulus, and ingrowth of the unmyelinated nerve ending that causes pain. Second, enhancing stability is achieved through the stiffening of the annular fiber from denaturation and shrinkage of the collagen. However, their mechanism is still unclear12). In order to satisfy the aforementioned rationale, the temperature must reach 45℃ in order to destroy the nociceptors and 60℃ to alter and shrink the collagen fiber9). However, there are some problems and disadvantages that are associated with this modality. It can induce degenerated intervertebral disc and disc herniation from the puncture site after the annulus puncture. Moreover, a probe tip that focuses heat was placed on the intra-annular area or nucleo-annular junction portion instead of the outer annulus with an ingrowth sensory-free nerve ending.
In our experiment, we decided to apply heat to the posterior annulus of the intervertebral disc of a cadaveric lumbar spine model by using a 1414-nm Nd : YAG laser, which was developed recently. A 1414-nm Nd : YAG laser, compared with Ho : YAG laser, is easier laser control, a smaller manufacturing fee, and equivocal effectiveness. Also compared with conventional NG : YAG laser, It is more safe because of shallow tissue penetration. This will now be described for thermal distributions.
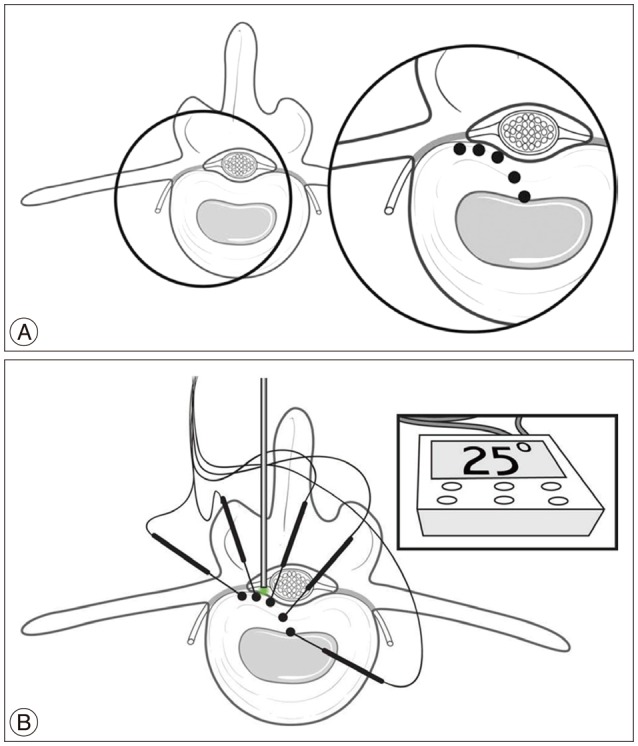
For this study, five discs from T12–L1 to L4–5 were obtained from 4 fresh adult human cadavers. The donor criteria excluded subjects with severe degeneration, such as fissuring, bridging osteophytes, history of spine trauma, infection, tumor, and any other bony diseases. The specimen was initially prepared by isolating the vertebral column from T12 to L5 and by cutting at T11–12 and L5–S1 disc level. It was prepared as en bloc, and the adjacent muscles and soft tissues were removed. Then, we removed the posterior elements of the spine by electric saw at the mid aspect of the pedicles. The cut vertebral column was placed in the water bath and maintained at a constant temperature of 37±1℃ by heat probe. In order to correct the temperature of the discs that are affected by the cerebrospinal fluid and blood circulation, we made some continuous artificial stir approximately 300 rpm via input and output circuit system of water bath (Fig. 1). For the measurement of the disc temperature distribution around the laser probe, 5 thermocouple probes [HYP1 (HYP0-30-1/2-T-G60-SMPW-M), Omega, Stamford, CT, USA] were found. Each probe was placed at 3 mm, 6 mm, and 9 mm laterally, and at 3 mm and 6 mm by depth as shown in Fig. 2.
Once all of the 5 probes constantly reached 37℃, we decided to apply heat at 600 µm fiber diameter of 1414 nm Nd : YAG laser system (Accuplasti, Lutronic, Goyang, Korea) placed at the midpoint of the posterior surface of the disc for 10 seconds. The pulse energy was kept at 200 mJ, while the pulse rate was set at 20 Hz. This recently developed 1414 nm Nd : YAG Laser's maximal pulse energy and rate was 600 mJ and 40 Hz. We used the selected parameter (200 mJ and 20 Hz) for our experiment dose without causing carbonization and vaporization of the cadaveric disc, and also observed gross shrinkage and hyalinization in the water bath during the preliminary study. We recorded and analyzed the temperature by using the multichannel data acquisition system (MV1000, Yokogawa Electric Corporation, Tokyo, Japan). The temperature measurement was done until the temperature of the thermocouple probe reached 37℃.
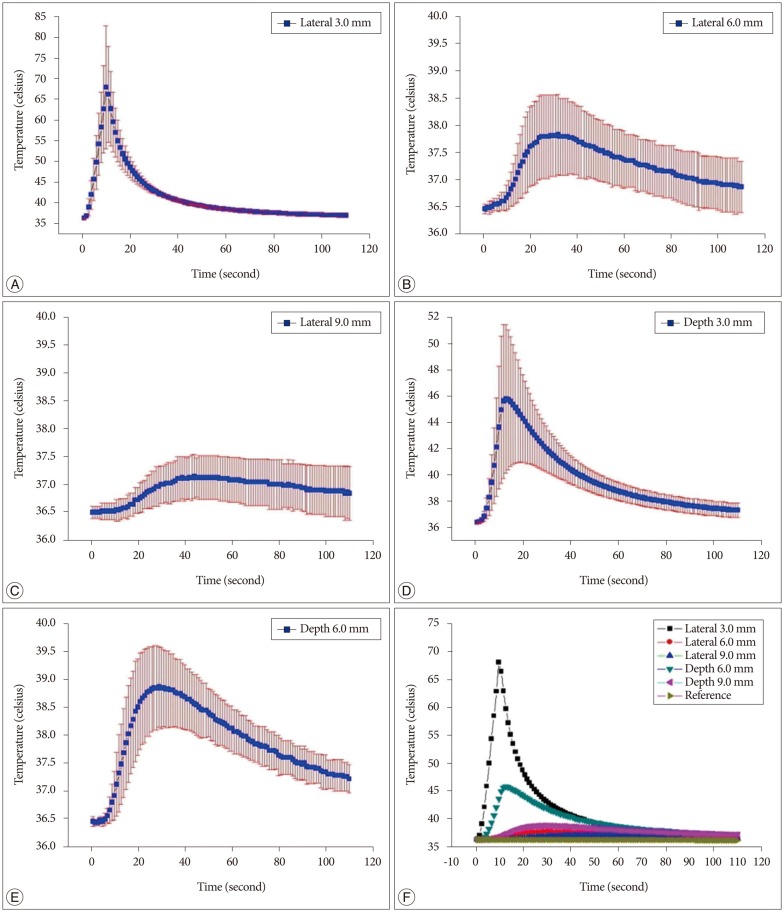
The average temperature recordings of the 5 probes from 20 specimens, according to time, can be observed from Fig. 3. The temperature would rise upon irradiation and fall slowly with the cessation of irradiation. The most distinctive elevation of the temperature was observed from probes located 3 mm away from the laser fiber tip, both in depth and lateral positions. The distribution curves, as measured across time, were quite varied depending on the location of the probes. The temperatures that were collected from the probes, which were closer to the laser tip, were higher than the temperatures from the probes that were located further away, while the probes that were placed laterally showed higher temperatures than the probes that were placed deeper.
The average maximum temperature recorded from the probes 3-mm and 6-mm deep from the laser tip were 47.0±4.3℃ and 38.7±0.9℃, and each measured at 13.6±0.8 seconds and 24.8±3.0 seconds, respectively. The average maximum temperature recorded from the probes 3 mm, 6 mm, and 9 mm lateral to the laser tip were 69.5±13.3℃, 37.8±0.7℃, and 37.2±0.3℃, and each recorded at 10.6±0.5 seconds, 26.6±3.4 seconds, and 38.8±4.4 seconds, respectively (Fig. 3).
The combination of the laser tip, 3-mm depth, and 16 levels (80%) showed a temperature above 45℃, which would result in a nociceptor ablation. Meanwhile, the relationship between the laser tip and laterality showed all levels at temperatures above 45℃, and 16 levels showed temperatures above 60℃, where collagen denaturation can be achieved. The temperatures from the lateral probes showed higher temperatures than the depth probes, which could be related to the characteristic of 1414 nm Nd : YAG laser, where the water showed much greater heat absorption potential than the human tissue.
The thermal procedures for discogenic LBP have been performed since the late 1990s, and the theoretical background was that the sources of discogenic pain were the nerve ingrowth and tissue regeneration into the annulus21). Therefore, achieving 45℃ or 60℃ through IDET or biacuplasty, where nociceptive ablation and collagen denaturation were possible, was the key point to cadaveric and animal experiments for thermal procedures4141518202224). Bono et al.4) reported that upon using IDET, the temperature above 60℃ was achieved within a 2-mm distance from the catheter, and the temperature above 45℃ was achieved at 9-mm to 14-mm distance away from the device. Shah et al.22) reported that by using IDET on the cadaveric model, they have managed to reach 55℃ in the outer annulus, while denaturation, shrinkage, and coalescence of the annular collagen were demonstrated on the histologic finding. However, Kleinstueck et al.15), reported that during their cadaveric study of IDET, temperatures above 60℃ were only achieved at approximately 1 mm to 2 mm around the catheter tip, while the temperatures adequate to damage nociceptor fibers were observed at 6 mm around the catheter tip without affecting the posterior annulus, where it is clinically relevant. These results have shown that the IDET, where the catheter is inserted and positioned in the inner border of the posterior annulus and then heated, has its limits in giving a direct thermal control on the free nerve endings from gray rami communicans and sinuvertebral nerves that innervated to the disc in cases of disc degeneration.
We have performed annuloplasty and used a laser for minimally invasive endoscopic procedures at the same time as one of thermal energy sources, and chose 1414 nm pulse wave Nd : YAG laser to evaluate the temperature distribution. For laser irradiation, an Nd : YAG laser with a wavelength of 1414 nm operating in the 200-mJ to 600-mJ range was used. We can observe the epidural space by epiduroscope and remove lesions, such as herniated disc, fibrosis, and adhesion, by laser. The laser power level was set to 200 mJ to 300 mJ (10 Hz to 20 Hz) for denervation and coagulation, and 300 mJ to 600 mJ (10 Hz to 20 Hz) for vaporization and ablation.
The adequate temperature for treatment in thermal therapy is considered to be above 60℃ at minimum, where collagen fibril shrinkage can be managed23). There have been a few studies describing the effects of thermal therapy on collagen. In their research on the effect of nonablative laser energy on in vitro rabbit femoropatellar joint capsule, Hayashi et al.10) observed the fusion of collagen and pyknotic nuclear changes in a fibroblast, and reported that there was no evidence of charring or removal of tissue.
High-power energy usage will result in laser that can induce tissue coagulation and charring, thereby leading to destruction. Therefore, it is essential to select a proper energy setting to ensure that the temperature, albeit above 60℃, is not high enough to damage the tissue. Jayasree et al.12) reported that when using a 1064-nm Nd : YAG laser for a disc decompression, it is ideal to use an initial delivery of 40 W laser and a reduced power of 10 W to 15 W thereafter. However, this setting is a parameter used in disc ablation, and it is too high to apply in laser annuloplasty. There were no investigations on specific parameters for such purpose, hence the pre-research was done to find the parameter that selectively induces shrinkage without gross annular charring or coagulation, as well as a pulse energy of 200 mJ, a pulse rate of 20 Hz, and a duration of 10 seconds.
The fact that the lateral surface of annulus have shown a higher temperature than the deeper portion of the annulus was quite a surprise, considering the laser directionality. Although the thermal properties of the tissue is determined by its water content, the spread of energy via thermal conductivity is influenced by the structure and complicated geometry of the tissue16). Also, transforaminal epidural lqser annuloplasty (TELA) actually is outside- in technique under epiduroscopic vision for the ablation of pain sources from the degenerative disc, while our experiment is close to IDET, which is actually inside posterior annulus-out ablating procedure without visionary assistance. That might be the reason why proper high temperature was conducted within 3 mm of laser tip. The temperature increase and distribution within a laser-irradiated tissue depends on the volume and thermal properties of the tissue25). In comparison to the former Nd : YAG laser, the 1414-nm wave Nd : YAG laser has a higher absorption rate of water. Furthermore, there was a higher heat conduction within the water bath than the annulus tissue, thereby showing a higher conduction laterally than longitudinally. Since it is a newly developed laser with a new wave length, further research about thermal distribution and histological change of tissue will be required.
In this study, the thermal distribution after laser irradiation in the annulus has shown a temperature increase in the relatively smaller radius, as compared to the previous studies on IDET and its temperatures4141520). Similar to the IDET, it is hard to apply heat to the entire annulus. However, it seems suitable for the treatment of focal discogenic pain source, such as annular tear or high intensity zone, by endoscopy or fluoroscopy. Unlike IDET, it will provide an extradiscal approach, thereby preserving a wide extent of healthy annulus without secondary damage due to a disc puncture. Furthermore, it can be applied to an endoscopic device that is compatible with percutaneous laser disc decompression or percutaneous endoscopic laser discectomy.
But our study has a several limitation. Even though depth of tissue penetration is 0.4 mm, by using a 1414-nm Nd : YAG laser, we didn't measured the temperature of surrounding root and dural sac which severe complication was arisen, if damaged. As one solution to this, there is a TELA procedure. The TELA has recently been introduced and considered as a new method for the treatment of discogenic LBP. The advantages of TELA as compared with back surgery, include relatively non-invasiveness, short operating time, reduction of risks related to general anesthesia, possibility of communication with the patient during the procedure (leading to reduced risks of accidental nerve damage), and easier access into ventral epidural space.
And the results of this study are limited through the use of cadaveric spine. Although our experiments were performed in a water bath to make closely similar situation of human spine as possible. However, this does not reflect the impact of soft tissue such as muscles and connective tissue around disc.
When measuring the temperature distribution of the annulus during laser annuloplasty with 1414-nm Nd : YAG laser, it could reach a sufficient temperature to ablate the nociceptor and induce collagen denaturation within a relatively narrow radius of 3 mm from the laser tip. It is also thought to be one of the most useful thermal procedures in clinical applications.
References
1. Alexandre A, Corò L, Paradiso R, Dall'aglio R, Alexandre AM, Fraschini F, et al. Treatment of symptomatic lumbar spinal degenerative pathologies by means of combined conservative biochemical treatments. Acta Neurochir Suppl. 2011; 108:127–135. PMID: 21107949.

2. Arnold PM, Robbins S, Paullus W, Faust S, Holt R, McGuire R. Clinical outcomes of lumbar degenerative disc disease treated with posterior lumbar interbody fusion allograft spacer : a prospective, multicenter trial with 2-year follow-up. Am J Orthop (Belle Mead NJ). 2009; 38:E115–E122. PMID: 19714280.
3. Bogduk N. The lumbar disc and low back pain. Neurosurg Clin N Am. 1991; 2:791–806. PMID: 1821758.

4. Bono CM, Iki K, Jalota A, Dawson K, Garfin SR. Temperatures within the lumbar disc and endplates during intradiscal electrothermal therapy : formulation of a predictive temperature map in relation to distance from the catheter. Spine (Phila Pa 1976). 2004; 29:1124–1129. discussion 1130-1131. PMID: 15131441.

5. Buric J, Pulidori M. Long-term reduction in pain and disability after surgery with the interspinous device for intervertebral assisted motion (DIAM) spinal stabilization system in patients with low back pain : 4-year follow-up from a longitudinal prospective case series. Eur Spine J. 2011; 20:1304–1311. PMID: 21279392.

6. Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for low back pain : a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine (Phila Pa 1976). 2009; 34:1094–1109. PMID: 19363455.
7. Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain : an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009; 34:1066–1077. PMID: 19363457.

8. Freeman BJ, Davenport J. Total disc replacement in the lumbar spine : a systematic review of the literature. Eur Spine J. 2006; 15(Suppl 3):S439–S447. PMID: 16862432.
9. Freeman BJ, Walters RM, Moore RJ, Fraser RD. Does intradiscal electrothermal therapy denervate and repair experimentally induced posterolateral annular tears in an animal model? Spine (Phila Pa 1976). 2003; 28:2602–2608. PMID: 14652477.

10. Hayashi K, Thabit G 3rd, Vailas AC, Bogdanske JJ, Cooley AJ, Markel MD. The effect of nonablative laser energy on joint capsular properties. An in vitro histologic and biochemical study using a rabbit model. Am J Sports Med. 1996; 24:640–646. PMID: 8883685.

11. Helm S, Hayek SM, Benyamin RM, Manchikanti L. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009; 12:207–232. PMID: 19165305.
12. Jayasree RS, Gupta AK, Bodhey NK, Mohanty M. Effect of 980-nm diode laser and 1064-nm Nd : YAG laser on the intervertebral disc--in vitro and in vivo studies. Photomed Laser Surg. 2009; 27:547–552. PMID: 19694506.

13. Kallewaard JW, Terheggen MA, Groen GJ, Sluijter ME, Derby R, Kapural L, et al. 15. Discogenic low back pain. Pain Pract. 2010; 10:560–579. PMID: 20825564.
14. Kapural L, Mekhail N, Hicks D, Kapural M, Sloan S, Moghal N, et al. Histological changes and temperature distribution studies of a novel bipolar radiofrequency heating system in degenerated and nondegenerated human cadaver lumbar discs. Pain Med. 2008; 9:68–75. PMID: 18254769.

15. Kleinstueck FS, Diederich CJ, Nau WH, Puttlitz CM, Smith JA, Bradford DS, et al. Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine. Spine (Phila Pa 1976). 2003; 28:1700–1708. discussion 1709. PMID: 12897495.

16. Knappe V, Frank F, Rohde E. Principles of lasers and biophotonic effects. Photomed Laser Surg. 2004; 22:411–417. PMID: 15671714.

17. Lee SH, Kang HS. Percutaneous endoscopic laser annuloplasty for discogenic low back pain. World Neurosurg. 2010; 73:198–206. discussion e133. PMID: 20860958.

18. Pauza K. Cadaveric intervertebral disc temperature mapping during disc biacuplasty. Pain Physician. 2008; 11:669–676. PMID: 18850031.
19. Peng B, Pang X, Wu Y, Zhao C, Song X. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain. 2010; 149:124–129. PMID: 20167430.

20. Petersohn JD, Conquergood LR, Leung M. Acute histologic effects and thermal distribution profile of disc biacuplasty using a novel water-cooled bipolar electrode system in an in vivo porcine model. Pain Med. 2008; 9:26–32. PMID: 18254764.

21. Saal JS, Saal JA. Management of chronic discogenic low back pain with a thermal intradiscal catheter. A preliminary report. Spine (Phila Pa 1976). 2000; 25:382–388. PMID: 10703114.

22. Shah RV, Lutz GE, Lee J, Doty SB, Rodeo S. Intradiskal electrothermal therapy : a preliminary histologic study. Arch Phys Med Rehabil. 2001; 82:1230–1237. PMID: 11552196.
23. Wall MS, Deng XH, Torzilli PA, Doty SB, O'Brien SJ, Warren RF. Thermal modification of collagen. J Shoulder Elbow Surg. 1999; 8:339–344. PMID: 10472007.

24. Wegener B, Rieskamp K, Büttner A, Habiyambere V, von Schultze-Pellangahr C, Schaffer V, et al. Experimental evaluation of the risk of extradiscal thermal damage in intradiscal electrothermal therapy (IDET). Pain Physician. 2012; 15:E99–E106. PMID: 22270753.
25. Zhao Y, Wang YP, Qiu GX, Zhao H, Zhang JG, Zhou X. Efficacy of the Dynamic Interspinous Assisted Motion system in clinical treatment of degenerative lumbar disease. Chin Med J (Engl). 2010; 123:2974–2977. PMID: 21162940.
Fig. 1
The apparatus used in the experiment. Using the above apparatus, experiments were conducted at a constant temperature (A : 1414 nm Nd : YAG laser system, B : thermal data loader, C : heating probe and circulating system, D : water bath, E : thermometer).
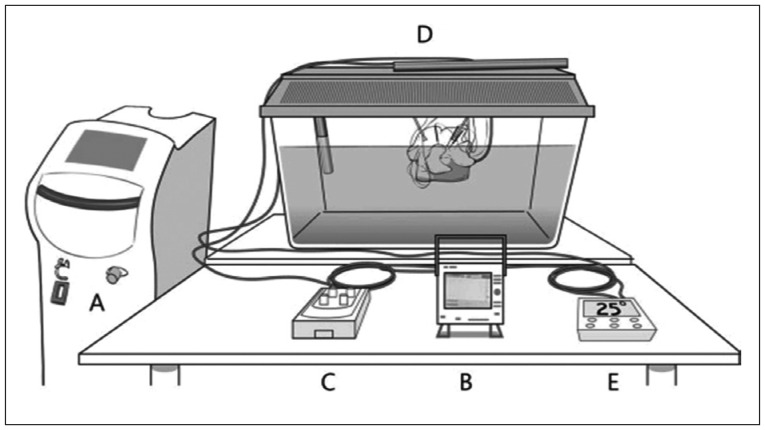
Fig. 2
Measurement of thermal distribution. A : Schematic illustration, each probe was placed 3, 6, and 9 mm laterally, and at depths of 3 and 6 mm from the laser fiber tip (dot). B : Experimental set up, 5 thermocouples probes [HYP1 (HYP0-30-1/2-T-G60-SMPW-M), Omega, Stamford, CT, USA] were located around the laser probe.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download