Abstract
Objective
To assess the impact of the complete resection of craniopharyngioma (CP) in adults on oncologic and functional outcomes.
Methods
We retrospectively analyzed 82 patients with CP who were surgically treated by the same neurosurgeon at our institution between January 1994 and December 2012.
Results
Gross total resection (GTR) was achieved in 71 patients (86.6%), near total resection (NTR) in 7 patients (8.5%), and subtotal resection (STR) in 3 patients (3.7%). The disease-specific overall survival rate was 100% with the exclusion of 2 surgery-related mortalities. The overall recurrence rate was 12.2% (10 of 82 patients), however the recurrence rate according to extent of resection (EOR) was 9.9% (7 of 71 patients) after GTR, 14.3% (1 of 7 patients) after NTR, and 66.7% (2 of 3 patients) after STR. The overall recurrence-free survival (RFS) rates at 5 and 10 years were 87.0% and 76.8%, respectively. Postoperatively, most patients (86.3%) needed hormone replacement for at least 1 hypothalamic-pituitary axis. Vision improved in 56.4% of the patients with preoperative abnormal vision, but deteriorated in 27.4% of patients. Hypothalamic dysfunction developed in 32.9% of patients. There were no significant differences in the risks of pituitary dysfunction, visual deterioration, or hypothalamic dysfunction between the groups with complete vs. incomplete removal. The overall rate of postoperative complications was 22.0%, which did not differ between groups (p=0.053).
Craniopharyngioma (CP) is a histologically benign but clinically aggressive epithelial tumor of the central nervous system. These tumors account for approximately 1% of all primary intracranial tumors in adults and 1-3% of intracranial tumors in children120). The annual incidence of CP ranges from 0.13-2 per 100000 persons without variance by sex or race142134).
CP is considered to arise from the embryonic rest cells of the craniopharyngeal duct or from metaplasia of the pituitary stalk or gland. Due to their tendency to grow in the sellar and suprasellar regions, these tumors have a very intricate relationship with important neurovascular structures, including the pituitary stalk, optic apparatus, and hypothalamus. A cure for CP may be achieved by complete resection of the tumor. CP demonstrates a much higher tendency to recur than other intracranial benign lesions, especially if any tumor tissue remains. The management of recurrent CP is much more difficult and often causes considerable morbidity. However, it is challenging to not only obtain surgical access to these regions, but also achieve their complete removal without morbidity. Furthermore, given the suspicion that the incidence of surgical morbidities markedly increases in patients who receive gross total resection (GTR) compared with patients who undergo subtotal resection (STR) with and without adjuvant therapy, conservative management has been recommended as the main surgical strategy by some authors13222329). However, there is still no conclusive evidence regarding the merits of either GTR or STR followed by radiation therapy20).
We consider complete tumor removal to be the most important principle for the treatment of CP. In our present study, we assess the effects of extent of resection (EOR) on recurrence, as well as postoperative endocrine, visual, and hypothalamic functions in adult CP patients with the aim of establishing the significance of GTR for the treatment of CP.
We retrospectively analyzed 82 patients with newly diagnosed CP who were surgically treated by the same neurosurgeon at our institution between January 1994 and December 2012. The Asan Medical Center Institutional Review Board granted a waiver for informed consent and approved this chart review (No. S2014-1069-0001). All diagnoses were based on histological examinations, except in one case of cyst aspiration alone, in which the neuroradiological features and the intra-operative findings were consistent with the diagnosis of CP. The medical records were reviewed to extract demographic information, presenting symptoms, operative findings, pre- and postoperative follow-up hormonal results, pre- and postoperative visual acuity (VA) and visual field (VF) findings, neurologic morbidities, complications, and recurrence.
Tumor characteristics (size, location, extent, calcification, and hypothalamic involvement) were evaluated using computed tomography (CT) and magnetic resonance imaging (MRI). Tumor size was determined by the maximal diameter on MRI (contrast enhanced T1-weighted imaging). Imaging data at presentation were available for 78 patients. Postoperative MRIs were examined 3 months after surgery, and then annual follow-up examinations were performed. At least one follow-up postoperative MRI was obtained for each of the 76 patients.
Complete resection was considered the primary surgical goal. Various microsurgical approaches were used according to the tumor size and extension. The pituitary stalk was transected during GTR in case the tumor was firmly attached to the stalk and could not be dissected from it. EOR was determined according to the intraoperative findings and confirmed on the first postoperative MRI. GTR was defined as the lack of residual tumor on intraoperative microscopic inspection and the absence of any residual mass or suspected tumorous enhancement on postoperative imaging. Near total resection (NTR) was defined as a remnant membrane or residual tumor area <1.5 cm2 on postoperative MRI. STR was defined as volumetric diminishment ≥90% and residual mass area ≥1.5 cm2 on MRI. Partial resection (PR) was defined as postoperative volumetric diminishment <90%. For the outcomes analysis, we categorized EOR into 2 groups : GTR and non-GTR (NTR, STR, or PR).
During the preoperative period, the basal levels of the pituitary-dependent hormonal axes were drawn in all patients. Postoperative hormonal status was examined within 1 week following surgery and reevaluated 3 months later. We analyzed the endocrine outcomes of all patients on follow-up examinations for more than 3 months. Postoperative endocrinopathies were defined as the development of any new mono- or polyhormonal anterior hypopituitarism or diabetes insipidus (DI).
Adrenocorticotrophic hormone (ACTH) deficiency was defined as a random morning serum cortisol level <4 µg/dL (100 nmol/L) in the absence of steroid therapy. Thyroid-stimulating hormone (TSH) deficiency was defined by low or inappropriately normal TSH level with thyroid hormone levels below the normal reference values. Gonadotrophin deficiency was diagnosed in the presence of low or inappropriately normal serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels combined with serum testosterone below the reference values in men or low serum estradiol along with history of oligo- or amenorrhea in women32). DI was diagnosed based on the presence of hypotonic (<300 mOsm/kg) polyuria (>3.5 L/24 h) together with a plasma sodium concentration of >145 mmol/L26). In many cases, growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels were omitted from our review of the medical records and not included in the analysis.
Preoperative VA and VF were evaluated in all patients, and postoperative follow-up tests were performed in 73 patients. Normal VA was defined as ≥0.8 in both eyes, and a preoperative decrease in VA was defined as <0.8 in either eye. VF defects were evaluated using Goldmann perimetry. To analyze visual outcomes, we compared the visual status on the last follow-up with the preoperative data and categorized the visual outcomes into the following 3 groups : 1) improved, which was defined as an increase in VA or decrease in VF defects in either eye (without any deterioration of VA or VF); 2) unchanged, which was defined as no change in either VA or VF; and 3) worsened, which was defined as the deterioration of either VA or VF.
Recurrence was diagnosed based on the radiological appearance (detection of tumor after GTR or tumor growth after non-GTR) with or without the associated signs and symptoms. Time to recurrence (TTR) was calculated from the date of initial surgery to the date of diagnosis of recurrence.
Statistical analyses were performed using SPSS (v.21.0; SPSS Inc., Chicago, IL, USA), and p<0.05 was used to indicate statistical significance. The evaluations were based on the number of patients with available data. Differences in the categorical variables were analyzed using the Fisher's exact test, and continuous variables were analyzed using the Mann-Whitney U-test. Variables that impacted the rates of recurrences and surgical complications were estimated using Cox proportional hazards model or logistic regression model. The recurrence-free curve was generated using the Kaplan-Meier method. For this analysis, the date of the first surgical procedure was defined as time zero.
This study included 53 male and 29 female patients (M : F ratio=1.83 : 1) with a median age of 42 years (range, 15-79 years). The median follow-up duration was 46 months (range, 5-169 months). The most common presenting symptom was visual disturbance (50 patients; 61.0%), followed by headache (33 patients; 40.2%), psychiatric manifestations (10 patients; 12.2%), hypopituitarism (9 patients; 11.0%), general weakness (7 patients; 8.5%), seizure (2 patients; 2.4%), decreased consciousness (2 patients; 2.4%), and incidental findings (2 patients; 2.4%). The median tumor diameter was 3.0 cm (range, 1.5-7.0 cm). On MRI, 18% of tumors were purely or predominantly solid, 20.5% were purely or predominantly cystic, and 61.5% were mixed. Calcifications were found in 57 patients (73.1%), and obstructive hydrocephalus was found in 20 patients (24.7%). The third ventricle was involved in 68 patients (82.9%). The tumors extended to the frontal lobe in 3 patients and to the posterior fossa in 2 patients (Fig. 1).
Sixty-two (76.5%) tumors were classified as adamantinomatous type and 19 (23.5%) were papillary type. There were significant differences in histological type and patient age at the time of presentation. The adamantinomatous type was more prevalent than the papillary type in younger patients : the median ages of the adamantinomatous and papillary groups were 34 years (range, 15-70) and 50 years (range, 31-79), respectively (p=0.019). No malignant histological features, such as atypical cell morphology or high mitotic activity, were identified.
Transcranial approaches were the primary treatment modalities, and thus were the most frequently used (n=79; 96.3%). Orbital craniotomies were mostly used in early cases of our series (n=22; 26.8%). The lateral subfrontal (n=31, 37.8%) or basal bifrontal interhemispheric (n=22, 26.8%) approaches were mainly performed on later cases according to tumor size and extension. In 2 patients (2.4%) with large tumors extending into the posterior fossa, staged operations were performed using combination of different surgical routes (the lateral subfrontal or interhemispheric approaches+the lateral suboccipital approach) (Fig. 2). The transsphenoidal approach (TSA) was performed on 2 sellar tumors. One patient with a large cystic tumor and severe obstructive hydrocephalus underwent endoscopic cyst aspiration and lateral subfrontal craniotomy. Another patient with a large tumor that extended into both the sellar and suprasellar area was treated with staged TSA and lateral subfrontal approach. Insertion of an Ommaya reservoir and aspiration of cystic fluid were performed on an elderly patient with a purely cystic tumor.
GTR was achieved in 71 patients (86.6%), NTR in 7 patients (8.5%), and STR in 3 patients (3.7%). One patient was treated by insertion of an Ommaya reservoir and cyst aspiration. Of the 11 patients with a remnant tumor, one patient with NTR and STR received stereotactic radiosurgery and radiation therapy as the adjuvant therapy, respectively. Nine patients were observed without any adjuvant treatment. There were no statistically significant differences between the GTR and non-GTR groups in terms of age (p=0.791), sex (p=0.312), tumor size (p=0.732), involvement of the hypothalamus (p=0.345), associated hydrocephalus (p=0.545), or histopathologic subtype (p=0.438). However, all tumors of the non-GTR group demonstrated calcification, and the difference between groups was statistically significant (p=0.029).
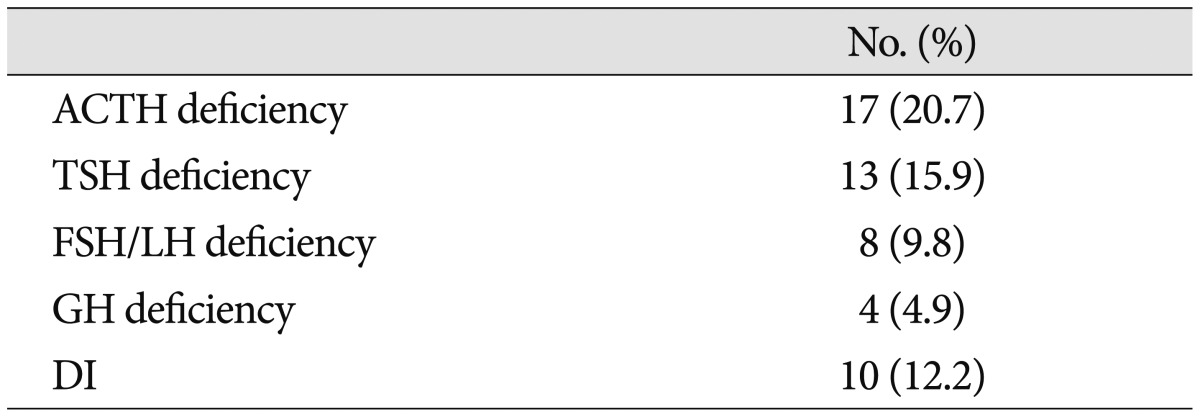
There were 2 surgery-related deaths (2.4%) that occurred during the first postoperative month. Both patients had undergone GTR, and each patient died of intracerebral hemorrhage (ICH) or sepsis due to catheter-related bacteremia, respectively. The most common postoperative complications were chronic subdural hematomas, meningitis, and communicating hydrocephalus, which were observed in 4 patients (4.9%), respectively. Less frequent complications included deep vein thrombosis and/or pulmonary embolism (3.7%), vasospasm (3.7%), cerebrospinal fluid leakage (2.4%), transient hemi- or paraparesis (2.4%), ICH (1.1%; caused by tumor bed bleeding), hemorrhagic infarction in both frontoparietal lobes caused by superior sagittal sinus thrombosis (1.1%), and pneumonia (1.1%). The overall frequency of postoperative complications was 22.0% (18 of 82 patients). There was no statistically significant correlation between the overall rates of postoperative complications and EOR (p=0.053). However, major complications, including ICH and infarction, were restricted to the GTR group, while the complications in the non-GTR group were relatively minor problems such as meningitis or chronic subdural hemorrhage (Table 1). Age (p=0.114), sex (p=0.343), tumor size (p=0.672), calcification (p=0.442), involvement of the hypothalamus (p=0.230), hydrocephalus (p=0.783), and stalk transection (p=0.520) did not significantly affect the development of complications according to the univariate analysis.
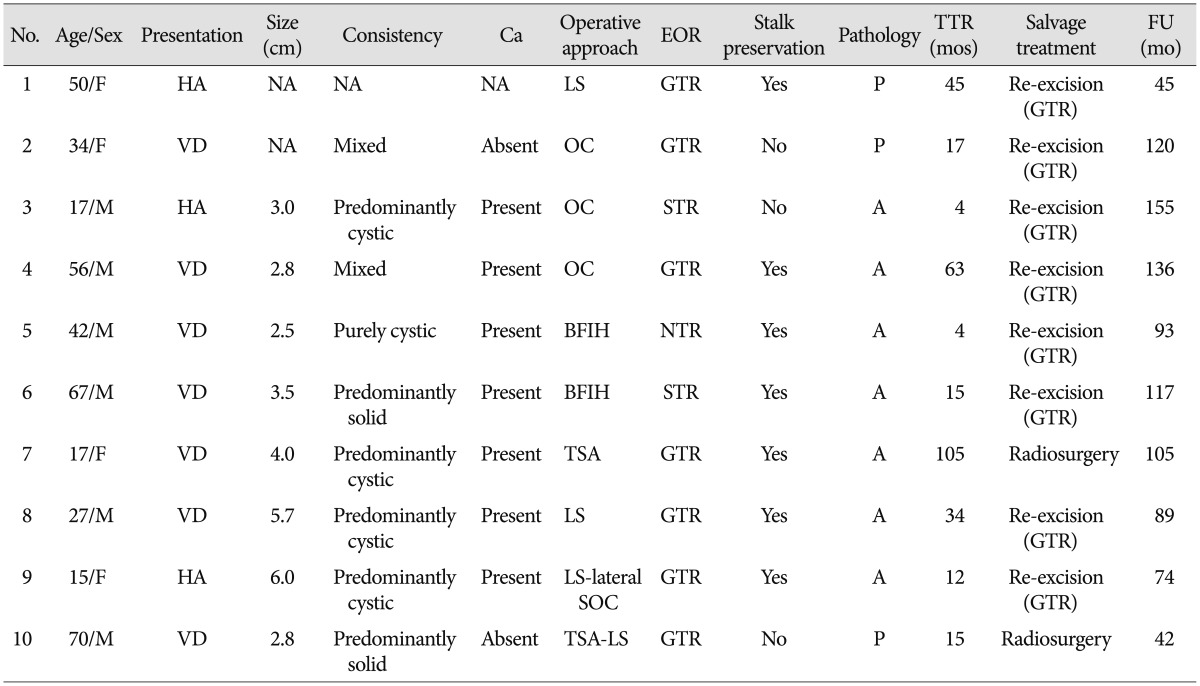
With the exclusion of 2 mortality cases, the disease-specific overall survival rate was 100%. The overall recurrence rate was 12.2% (10 of 82 patients). However, the recurrence rates according to EOR were significantly different : 9.9% (7 of 71 patients) after GTR, 14.3% (1 of 7 patients) after NTR, and 66.7% (2 of 3 patients) of patients after STR developed recurrence. The median TTR was 16 months (range, 4-105 months). The overall recurrence-free survival (RFS) rates at 5 and 10 years were 87.0% and 76.8%, respectively (Fig. 3). In the GTR group, the RFS rates at 1, 3, 5, and 10 years were 98.4%, 92.5%, 89.8%, and 79.2%, respectively, whereas the RFS rates at 1, 3, and 5 years in the non-GTR group were 81.8%, 72.7%, and 72.7%, respectively (p=0.020 according to the log-rank test) (Fig. 4). There was a trend toward faster recurrence in the non-GTR group (median TTR=4 months) in comparison with the GTR group (median TTR=34 months). According to the univariate analysis, only non-GTR treatment was significantly related to tumor recurrence (HR=4.788; 95% CI=1.108-20.681; p=0.036). Age, sex, tumor size, involvement of the hypothalamus, calcification, and pathologic subtype did not significantly affect recurrence. There was a statistical trend toward recurrence in patients with pituitary stalk preservation (21.2% of patients with stalk preservation vs. 6.4% of patients with stalk transection), although this finding was not statistically significant (p=0.069). In the non-GTR group, tumors did not progress in patients who received adjuvant therapy. As a salvage treatment, 8 patients underwent reexcision of the recurrent tumor, and 1 patient received radiosurgery. The clinical features of recurrence cases are summarized in Table 2.
The median follow-up duration of our study was 46 months (range, from 5-169 months), which is relatively shorter than previous reports. Our present analysis included 9 patients who were lost on follow-up after <1 year. Considering the possibility that a short follow-up duration affected favorable outcome on tumor recurrence, we reevaluated the RFS after excluding these 9 patients. In this group (n=73), the median follow-up duration was 57 months. The overall RFS rates at 5 and 10 years were 86.8% and 76.6%, respectively. The pattern of the Kaplan-Meier curve was similar to the graph of the original group. The detailed RFS rates according to the EOR were identical to those of the original group (Fig. 5).
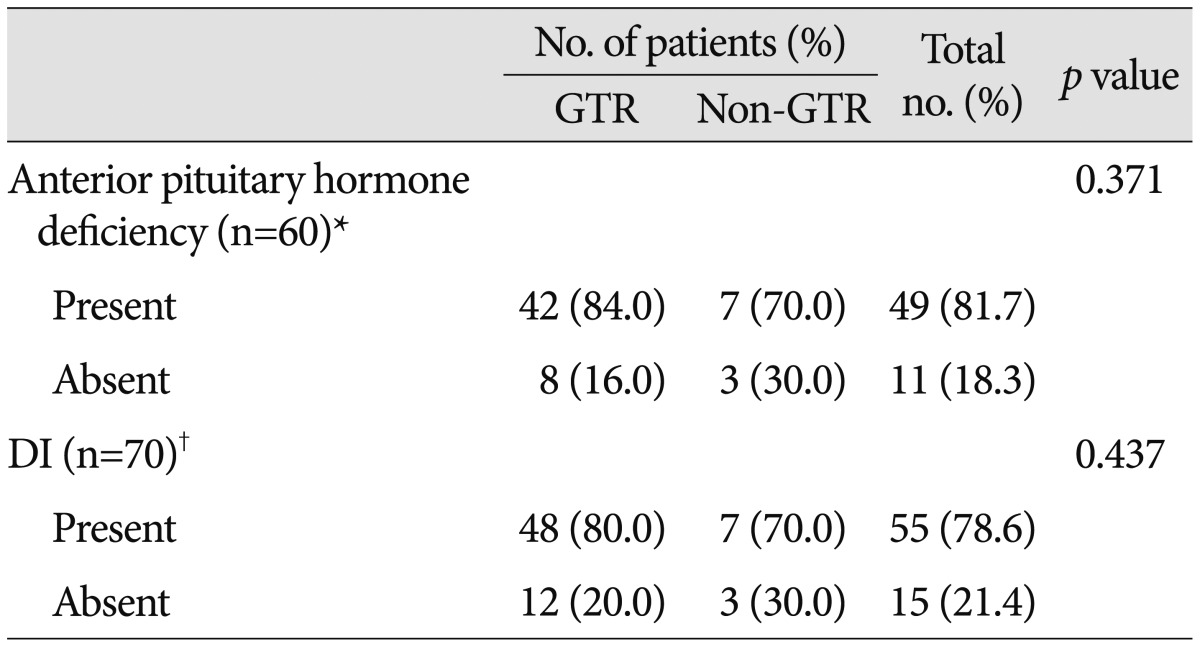
The pituitary stalk was preserved in 26 patients (31.7%), partially preserved (defined as when the stalk was curetted to remove the tumor that adhered to the stalk) in 7 patients (8.5%), and transected in 49 patients (59.8%). The pituitary stalk was transected more frequently in the GTR group than the non-GTR group (p=0.011). Preoperatively, overall anterior pituitary hormone deficiency and DI were diagnosed in 20 (24.4%) and 10 patients (12.2%), respectively (Table 3). Postoperatively, hormone replacement was needed in 69 patients (86.3%) and DI was documented in 65 patients (81.3%). After surgery, anterior pituitary hormone deficiency and DI newly developed in 49 (81.7%) and 55 patients (78.6%), respectively. The endocrine outcomes according to EOR are summarized in Table 4. Hormone replacement was still required in 69.2% of patients with pituitary stalk preservation and 57.1% of patients with the partial stalk preservation. GTR did not significantly increase the risk of endocrinopathies compared with non-GTR (p=0.371 for anterior pituitary hormone deficiency; p=0.437 for DI).
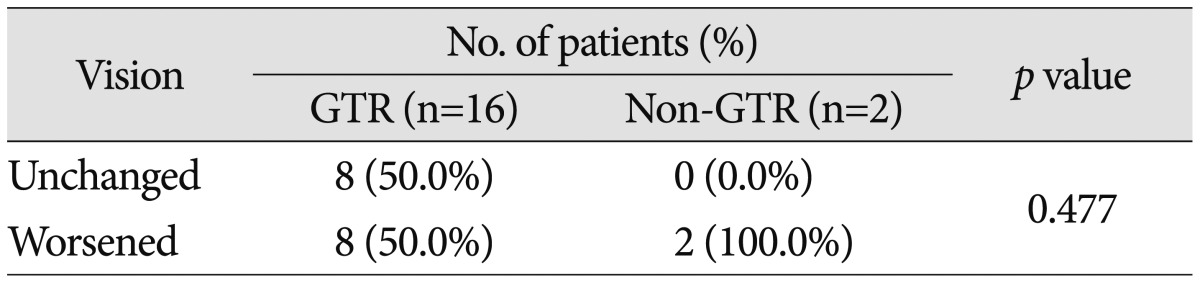
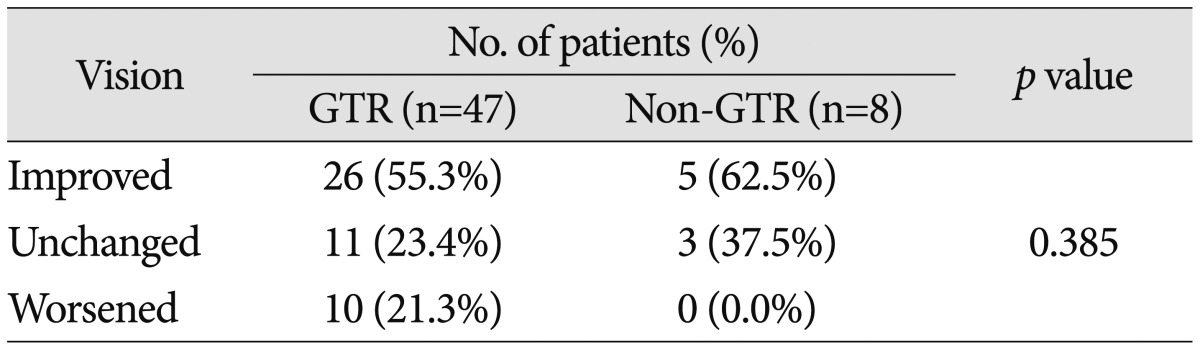
Preoperatively, 58 patients (70.7%) demonstrated a decrease in VA and 61 (74.4%) had a VF defect. The most common type of VF defect was bitemporal hemianopsia (25 patients; 30.5%). The other observed visual field abnormalities included monocular visual loss with unitemporal hemianopsia (15 patients), homonymous hemianopsia (12 patients), unitemporal hemianopsia (7 patients), and monocular visual loss (2 patients). After surgery, 10 of the 18 patients with normal preoperative VA and VF (55.6%) experienced either worsening of VA or VF. Of the 55 patients with abnormal VA or VF, postoperative vision improved in 31 patients (56.4%), remained unchanged in 14 patients (25.5%), or deteriorated in 10 patients (18.2%). The detailed visual outcomes according to EOR are summarized in Table 5, 6. The difference in visual outcomes between the GTR and non-GTR groups was not significant (p=0.477 in patients with preoperative normal vision; p=0.385 in patients with preoperative abnormal vision).
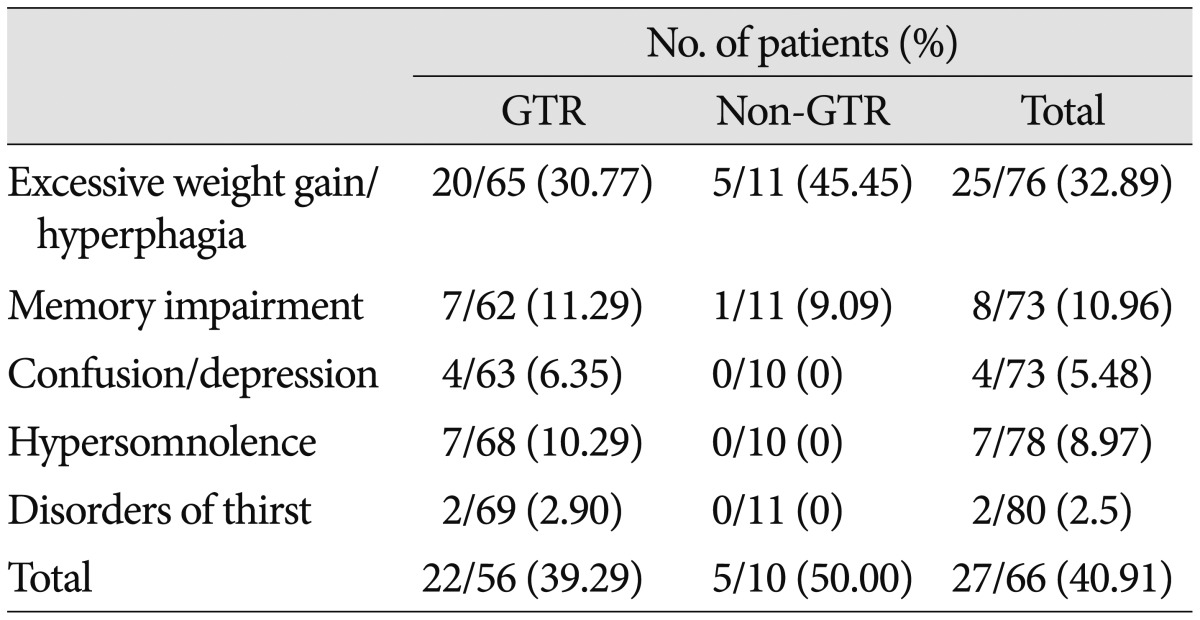
Hypothalamic dysfunction-which includes hyperphagia, memory or cognitive impairment, sleep-wake cycle disorder, and thirst disorder-newly appeared in 27 of 66 patients (40.91%) after surgery. Details regarding hypothalamic dysfunction according to the EOR are summarized in Table 7. Excessive weight gain (defined as a weight increase ≥5 kg over 1 month) was observed in approximately one-third of all patients. Memory impairment, in the form of anterograde amnesia, developed in approximately 10% of patients. There was no significant difference in the overall incidence of hypothalamic dysfunction between the GTR and non-GTR groups (p=0.729). However, psychiatric symptoms (4 patients), such as personality change, confusion, and depression, sleep-wake cycle disorder (7 patients), and thirst disorder (2 patients) were only observed in the GTR group.
The anatomical relationships between neurovascular structures are often distorted by CP, and the surgical cleavage planes are often indistinguishable without careful consideration. Although CP is a benign tumor with well-demarcated margins, it often firmly adheres to the surrounding neurovascular structures. Careful inspection of the pathoanatomy of the tissue surrounding the CP not only enabled us to delineate the tumor margins, but usually revealed the arachnoid or gliotic planes between the tumors and surrounding neurovascular structures such as the optic apparatus, internal carotid artery, and even the hypothalamus. Our surgical philosophy involves first attempting the complete resection of CP, provided this is deemed to be safe. Then, in cases of large tumors that extend into multiple compartments, we perform a staged operation using a combination of different surgical routes in order to achieve GTR. CPs were frequently found to arise from the pituitary stalk and recur around the stalk. In our early series, we made an effort to preserve the pituitary stalk, if possible. However, our preanalysis showed that recurrence seems to be associated with pituitary stalk preservation. The recurrence rates of patients with stalk preservation and patients with stalk transection were 21.2% and 6.4%, respectively. These results did not meet the level of statistical significance (p=0.069), however they could have been underestimated due to the small number of patients. In our later series, we resolutely transected the stalk in order to attain GTR if the tumors could not be dissected from the stalk. In cases where the tumors strongly adhered to the vital structures, such as the brainstem, we made every effort to restrict the volume of the tumor to the smallest size possible.
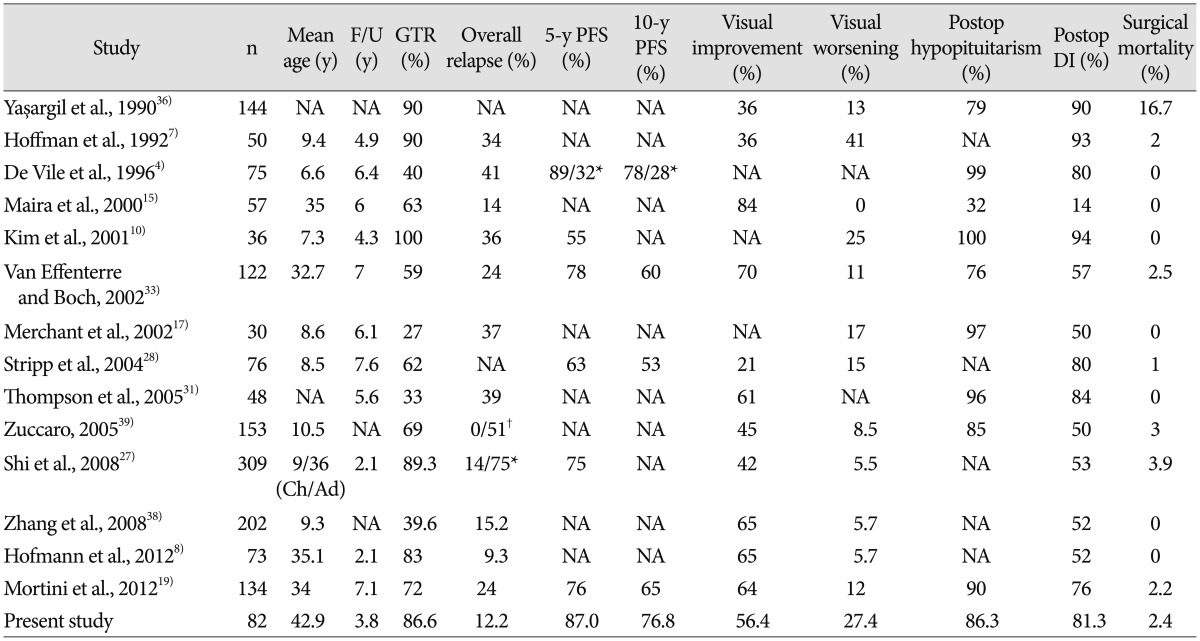
In our current study, GTR was accomplished in 86.6% of patients. The overall disease-specific survival was 100% after excluding 2 mortality cases, which occurred in the immediate postoperative period. The overall RFS rates at 5 and 10 years were 87.0% and 76.8%, respectively. Given the considerable differences between studies in terms of the treatment modalities, EOR, and recurrence rates, an accurate comparative analysis of the studies with respect to surgical outcomes is difficult. However, the rate of tumor recurrence in patients who underwent GTR varied to a lesser extent among studies. For instance, Karavitaki et al.9) reported no recurrence in any of the 19 patients included in their study. The recurrence rate was 7.7% in the study reported by Caldarelli et al.2), 11% in the studies reported by both Fahlbusch et al.5) and Kitano and Taneda12), and 13% in the studies reported by both Van Effenterre and Boch33) and Shi et al.27). In our study, recurrence after GTR developed in 9.9% of all cases, and the overall rate of recurrence was 12.2%.
It has been well demonstrated that EOR is the most significant factor associated with recurrence520333637). Our analysis also indicated that EOR was the only factor able to predict recurrence; the rate of recurrence of the GTR group was lower than that of the non-GTR group (9.9% vs. 27.3%; p=0.036), and TTR in the non-GTR group was much faster than the GTR group (median TTR, 4 months vs. 34 months). The re-excision of recurrent tumors is very challenging because the recurrent tumors are locally invasive and usually adhere to adjacent critical neurovascular structures; both of these features often result in high levels of surgical morbidity. Therefore, if possible, we recommend performing GTR during the initial surgical intervention : this will increase the likelihood of positive oncologic outcomes and reduce the likelihood of long-term morbidities.
In our current series, adjuvant therapy was performed on only 2 of 11 patients who underwent non-GTR treatment (1 radiosurgery and 1 conventional radiation therapy). No recurrence in either case was observed during the 25- and 36-months follow-up periods. We were unable to verify the statistical correlation between adjuvant therapy and recurrence due to the small sample size in the current study. However, it has been well established that subsequent radiation therapies following non-GTR significantly decrease the recurrence rate1316222324).
It has been proposed that morbidities such as endocrine, visual, and hypothalamic dysfunctions are markedly more prevalent in patients who receive GTR compared with those who receive STR with or without radiotherapy (stereotactic radiosurgery or conventional radiation therapy)29). Furthermore, it has been reported that STR with radiotherapy might reduce the tumor recurrence rate to levels comparable to those found in patients who receive GTR. Thus, STR with radiotherapy has been emphasized as a predominant surgical strategy by some authors361825). However, in our present analysis, there was no statistical significance found between the GTR and non-GTR groups in terms of postoperative endocrine, visual, hypothalamic dysfunctions. In other words, the rates of functional morbidities in the patients who received GTR were comparable to those who received STR. Our results were also comparable to previously reported endocrine and visual outcomes (Table 8).
The retrospective study by Karavitaki et al.9), which involved the analysis of 121 CPs in children and adults, failed to reveal a significant difference in the probability of new endocrinopathies, functional outcomes, and surgical morbidities depending on the treatment modality (GTR vs. GTR+radiotherapy vs. STR vs. STR+radiotherapy). However, they emphasized that visual deterioration was higher among patients who received STR alone. Although Kim et al.11) reported that the rate of short-term visual deterioration was higher in patients with GTR than those with STR (either with or without radiotherapy), the differences were not significant. However, after a long-term follow-up, the patients who received STR alone (51%) demonstrated a much higher rate of visual deterioration than patients with GTR (15%) or STR+radiotherapy (19%; p<0.001). This difference was attributed to a higher rate of tumor recurrence in patients who received STR alone. Meanwhile, Sughrue et al.29) reported that all patients with CPs who received radiotherapy experienced higher rates of visual compromise than the patients who received only surgery. Other studies also identified higher rates of mortality and morbidity among patients who underwent surgeries for CP recurrence53035).
In summary, the high rate of morbidity for endocrine, visual, and neuropsychiatric dysfunctions might not be limited to patients who undergo GTR. This possibility should be considered when comparing the overall outcomes of GTR and STR. In addition, when the tumor is firmly attached to critical neurovascular structures and thus incomplete tumor removal is inevitable, maximal decompression of the tumor might reduce the side effects associated with radiotherapy.
CP is a histologically benign tumor, although it often shows locally aggressive behaviors. Thus, cure is possible by complete excision. Incomplete resection is associated with a high recurrence rate, further morbidity and mortality from tumor regrowth, and risk of repeated surgery. Therefore, we propose that the goal of surgery should be complete tumor excision, especially at the first surgery. Then, careful follow-up is required and active treatment like re-excision or radiotherapy should be performed when recurrence develops.
References
1. Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998; 89:547–551. PMID: 9761047.

2. Caldarelli M, Massimi L, Tamburrini G, Cappa M, Di Rocco C. Long-term results of the surgical treatment of craniopharyngioma : the experience at the Policlinico Gemelli, Catholic University, Rome. Childs Nerv Syst. 2005; 21:747–757. PMID: 15995885.

3. Crowley RK, Sherlock M, Agha A, Smith D, Thompson CJ. Clinical insights into adipsic diabetes insipidus : a large case series. Clin Endocrinol (Oxf). 2007; 66:475–482. PMID: 17371462.
4. De Vile CJ, Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, et al. Management of childhood craniopharyngioma : can the morbidity of radical surgery be predicted. J Neurosurg. 1996; 85:73–81. PMID: 8683285.

5. Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas : experience with 168 patients. J Neurosurg. 1999; 90:237–250. PMID: 9950494.
6. Habrand JL, Saran F, Alapetite C, Noel G, El Boustany R, Grill J. Radiation therapy in the management of craniopharyngioma : current concepts and future developments. J Pediatr Endocrinol Metab. 2006; 19(Suppl):389–394. PMID: 16700315.
7. Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI. Aggressive surgical management of craniopharyngiomas in children. J Neurosurg. 1992; 76:47–52. PMID: 1727168.

8. Hofmann BM, Höllig A, Strauss C, Buslei R, Buchfelder M, Fahlbusch R. Results after treatment of craniopharyngiomas : further experiences with 73 patients since 1997. J Neurosurg. 2012; 116:373–384. PMID: 21942724.

9. Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, Ansorge O, et al. Craniopharyngiomas in children and adults : systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005; 62:397–409. PMID: 15807869.

10. Kim SK, Wang KC, Shin SH, Choe G, Chi JG, Cho BK. Radical excision of pediatric craniopharyngioma : recurrence pattern and prognostic factors. Childs Nerv Syst. 2001; 17:531–536. discussion 537PMID: 11585327.

11. Kim YH, Kim CY, Kim JW, Kim YH, Han JH, Park CK, et al. Longitudinal analysis of visual outcomes after surgical treatment of adult craniopharyngiomas. Neurosurgery. 2012; 71:715–721. PMID: 22668887.

12. Kitano M, Taneda M. Extended transsphenoidal surgery for suprasellar craniopharyngiomas : infrachiasmatic radical resection combined with or without a suprachiasmatic trans-lamina terminalis approach. Surg Neurol. 2009; 71:290–298. discussion 298PMID: 18291485.

13. Kobayashi T, Tanaka T, Kida Y. Stereotactic gamma radiosurgery of craniopharyngiomas. Pediatr Neurosurg. 1994; 21(Suppl):69–74. PMID: 7841081.

14. Lopez-Serna R, Gómez-Amador JL, Barges-Coll J, Nathal-Vera E, Revuelta-Gutiérrez R, Alonso-Vanegas M, et al. Treatment of craniopharyngioma in adults : systematic analysis of a 25-year experience. Arch Med Res. 2012; 43:347–355. PMID: 22824214.

15. Maira G, Anile C, Albanese A, Cabezas D, Pardi F, Vignati A. The role of transsphenoidal surgery in the treatment of craniopharyngiomas. J Neurosurg. 2004; 100:445–451. PMID: 15035280.

16. Manaka S, Teramoto A, Takakura K. The efficacy of radiotherapy for craniopharyngioma. J Neurosurg. 1985; 62:648–656. PMID: 3989587.

17. Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, et al. Craniopharyngioma : the St. Jude Children's Research Hospital experience 1984-2001. Int J Radiat Oncol Biol Phys. 2002; 53:533–542. PMID: 12062594.

18. Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006; 104(2 Suppl):94–102. PMID: 16506496.

19. Mortini P, Gagliardi F, Boari N, Losa M. Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit Rev Oncol Hematol. 2013; 88:514–529. PMID: 23932582.

20. Pekmezci M, Louie J, Gupta N, Bloomer MM, Tihan T. Clinicopathological characteristics of adamantinomatous and papillary craniopharyngiomas : University of California, San Francisco experience 1985-2005. Neurosurgery. 2010; 67:1341–1349. discussion 1349PMID: 20871436.

21. Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst. 2005; 21:622–627. PMID: 15965669.

22. Prasad D, Steiner M, Steiner L. Gamma knife surgery for craniopharyngioma. Acta Neurochir (Wien). 1995; 134:167–176. PMID: 8748777.

23. Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, et al. Craniopharyngioma--a long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993; 26:1–10. PMID: 8438080.

24. Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol. 1993; 27:13–21. PMID: 8327728.

25. Scarzello G, Buzzaccarini MS, Perilongo G, Viscardi E, Faggin R, Carollo C, et al. Acute and late morbidity after limited resection and focal radiation therapy in craniopharyngiomas. J Pediatr Endocrinol Metab. 2006; 19(Suppl):399–405. PMID: 16700317.
27. Shi XE, Wu B, Fan T, Zhou ZQ, Zhang YL. Craniopharyngioma : surgical experience of 309 cases in China. Clin Neurol Neurosurg. 2008; 110:151–159. PMID: 18063470.
28. Stripp DC, Maity A, Janss AJ, Belasco JB, Tochner ZA, Goldwein JW, et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004; 58:714–720. PMID: 14967425.

29. Sughrue ME, Yang I, Kane AJ, Fang S, Clark AJ, Aranda D, et al. Endocrinologic, neurologic, and visual morbidity after treatment for craniopharyngioma. J Neurooncol. 2011; 101:463–476. PMID: 20535527.

30. Symon L. Microsurgery of the hypothalamus with special reference to craniopharyngioma. Neurosurg Rev. 1983; 6:43–49. PMID: 6657077.

31. Thompson D, Phipps K, Hayward R. Craniopharyngioma in childhood : our evidence-based approach to management. Childs Nerv Syst. 2005; 21:660–668. PMID: 15959733.

32. Toogood AA, Stewart PM. Hypopituitarism : clinical features, diagnosis, and management. Endocrinol Metab Clin North Am. 2008; 37:235–261. PMID: 18226739.
33. Van Effenterre R, Boch AL. Craniopharyngioma in adults and children : a study of 122 surgical cases. J Neurosurg. 2002; 97:3–11. PMID: 12134929.
34. Wang KC, Hong SH, Kim SK, Cho BK. Origin of craniopharyngiomas : implication on the growth pattern. Childs Nerv Syst. 2005; 21:628–634. PMID: 16059733.

35. Wisoff JH. Surgical management of recurrent craniopharyngiomas. Pediatr Neurosurg. 1994; 21(Suppl):108–113. PMID: 7841068.

36. Yaşargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990; 73:3–11. PMID: 2352020.
37. Yi S, Yang KH, Kim DS, Choi JU. Craniopharyngiomas : predictive factors of recurrence. J Korean Neurosurg Soc. 2002; 32:189–195.
38. Zhang YQ, Ma ZY, Wu ZB, Luo SQ, Wang ZC. Radical resection of 202 pediatric craniopharyngiomas with special reference to the surgical approaches and hypothalamic protection. Pediatr Neurosurg. 2008; 44:435–443. PMID: 19018151.

39. Zuccaro G. Radical resection of craniopharyngioma. Childs Nerv Syst. 2005; 21:679–690. PMID: 16133275.

Fig. 1
Preoperative magnetic resonance images of a 21-year-old male patient with seizure showing a sellar and suprasellar mass composed of solid and huge cystic portions extending into the right frontal lobe, which is compatible with craniopharyngioma.
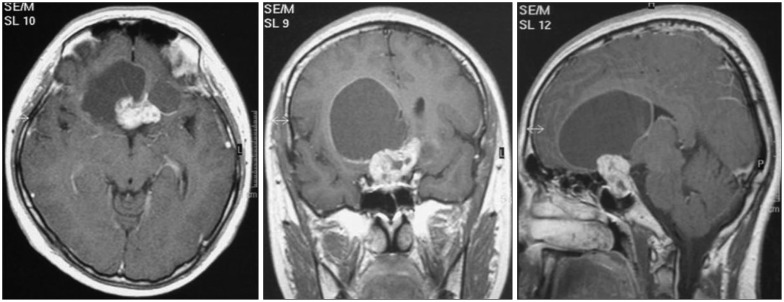
Fig. 2
Magnetic resonance images of a 15-year-old female patient with headache showing a large complex cystic mass at the prepontine cistern with extension into the suprasellar area. She underwent a staged operation via the lateral suboccipital approach and lateral subfrontal approach in order to achieve gross total resection.
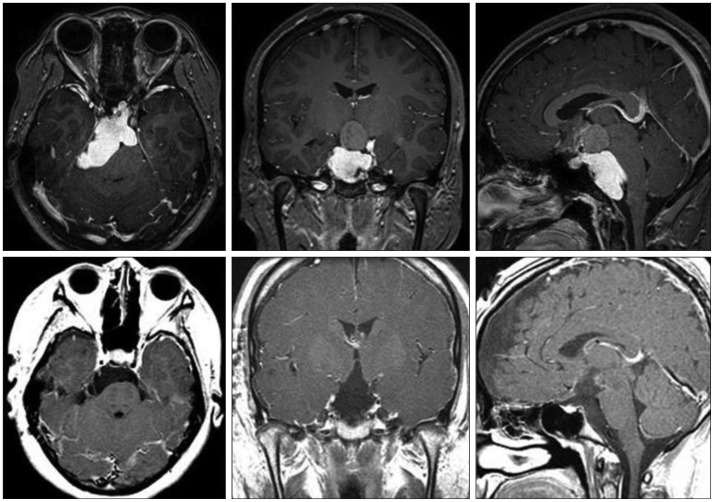
Fig. 3
Kaplan-Meier recurrence-free survival curve of patients who underwent surgery for craniopharyngioma.
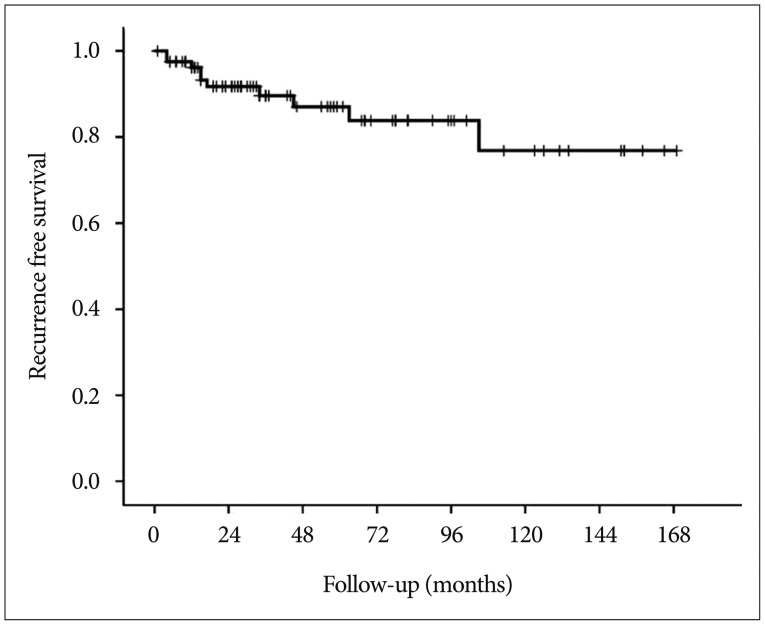
Fig. 4
Kaplan-Meier curves comparing recurrence-free survival between the gross total resection (GTR) and non-GTR groups.
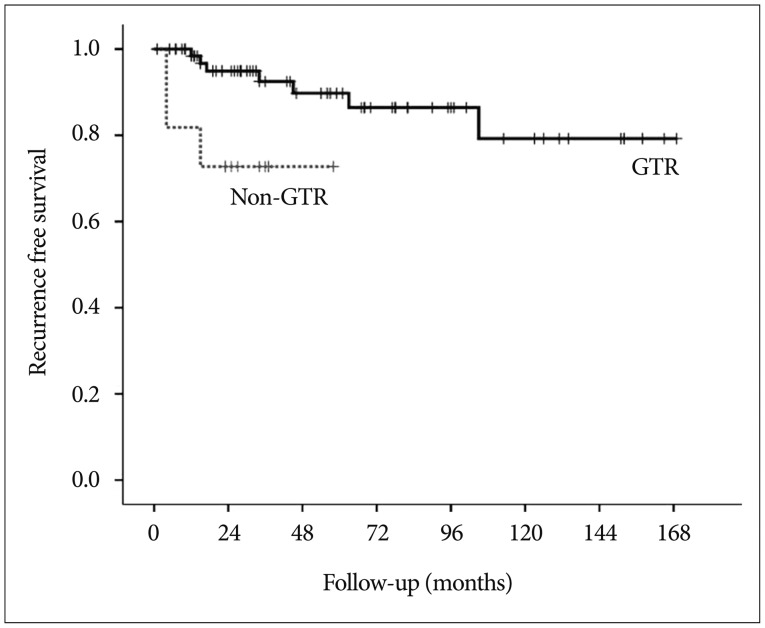
Fig. 5
Kaplan-Meier recurrence-free survival curves of the patients who followed up more than 1 year. A : Graph shows the overall recurrence-free survival rates. B : Graph compares recurrence-free survival between the GTR and non-GTR groups. For patients with follow-up ≥12 months (n=73). GTR : gross total resection.
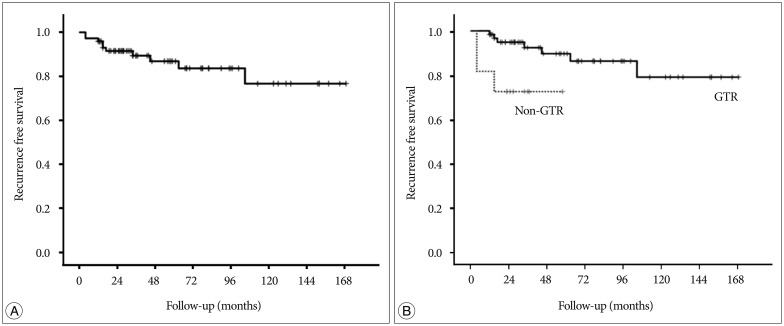
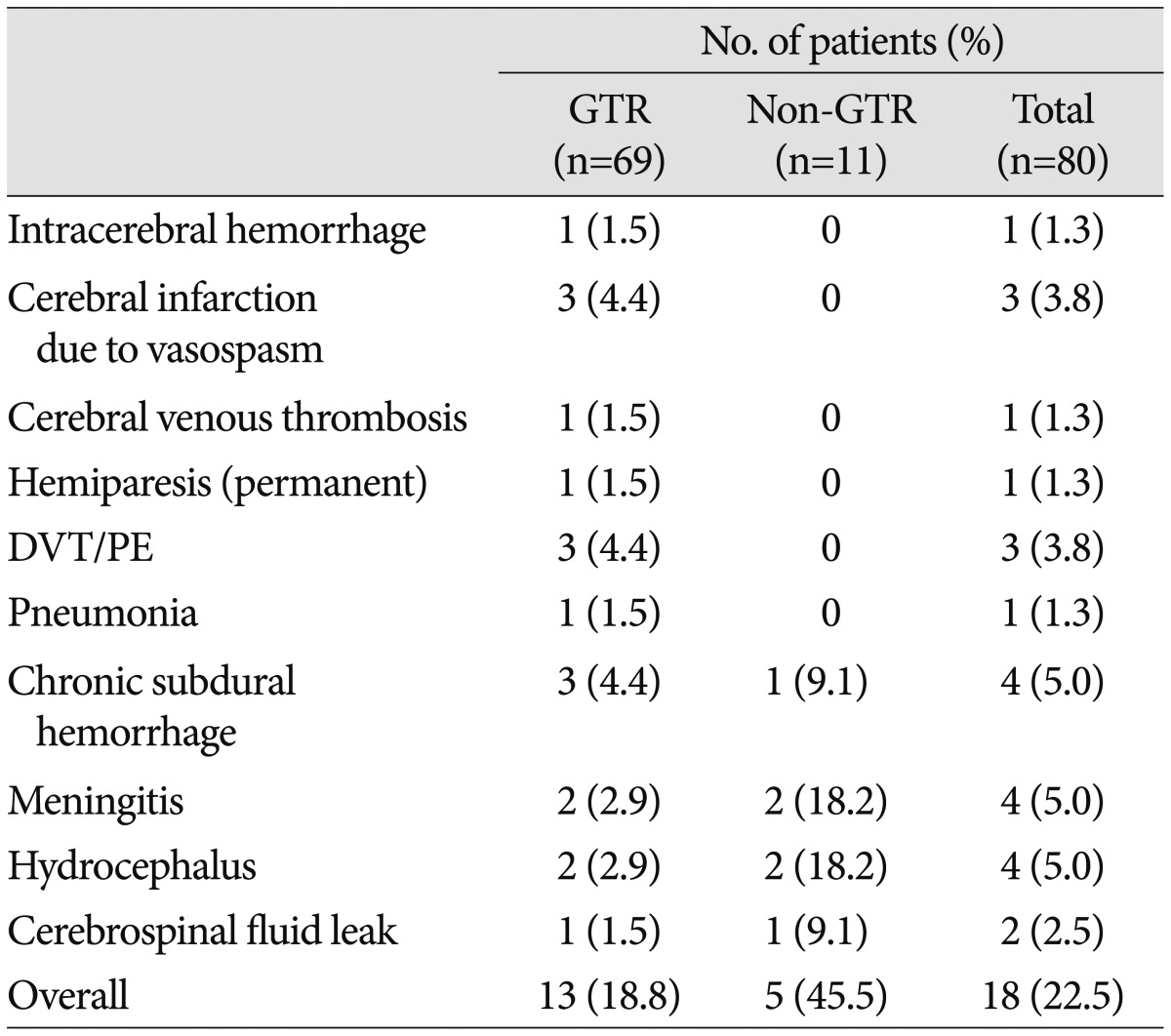
Table 2
Summary of recurrence cases : tumor characteristics, EOR, time to recurrence, and salvage treatment
A : adamantinomatous, BFIH : basal bifrontal interhemispheric, Ca : calcification, EOR : extent of resection, GTR : gross total resection, HA : headache, LS : lateral subfrontal, mos : months, NA : not applicable, NTR : near total resection, OC : orbitocranial, P : papillary, SOC : suboccipital craniotomy, STR : subtotal resection, TSA : transsphenoidal approach, TTR : time to recurrence VD : visual disturbance




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download