Abstract
Objective
Chronic subdural hematoma (CSDH) is a rare complication of unruptured aneurysm clipping surgery. The purpose of this study was to identify the incidence and risk factors of postoperative CSDH after surgical clipping for unruptured anterior circulation aneurysms.
Methods
This retrospective study included 518 patients from a single tertiary institute from January 2008 to December 2013. CSDH was defined as subdural hemorrhage which needed surgical treatment. The degree of brain atrophy was estimated using the bicaudate ratio (BCR) index. We used uni- and multivariate analyses to identify risk factors correlated with CSDH.
Results
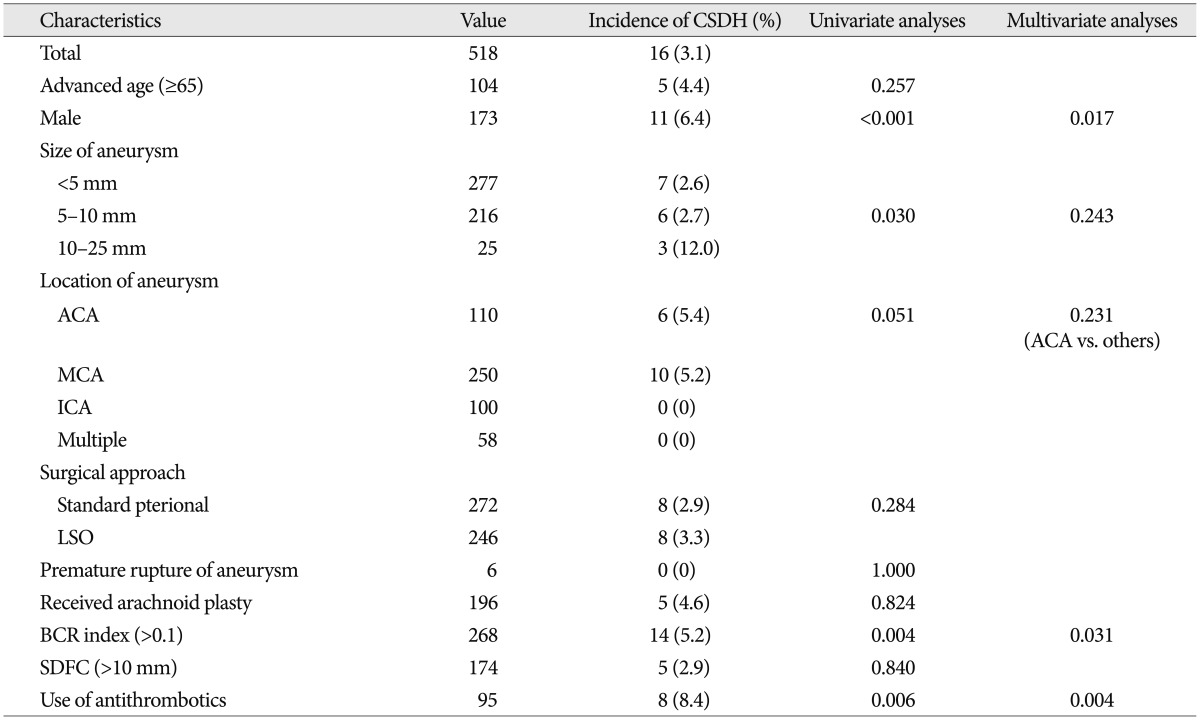
Sixteen (3.1%) patients experienced postoperative CSDH that required burr hole drainage surgery. In univariate analyses, male gender (p<0.001), size of aneurysm (p=0.030), higher BCR index (p=0.004), and the use of antithrombotic medication (p=0.006) were associated with postoperative CSDH. In multivariate analyses using logistic regression test, male gender [odds ratio (OR) 4.037, range 1.287-12.688], high BCR index (OR 5.376, range 1.170-25.000), and the use of antithrombotic medication (OR 4.854, range 1.658-14.085) were associated with postoperative CSDH (p<0.05). Postoperative subdural fluid collection and arachnoid plasty were not showed statistically significant difference in this study.
Chronic subdural hematoma (CSDH) is a rare complication of unruptured aneurysm clipping surgery5,12). Because CSDH signs and symptoms may appear slowly, development of postoperative CSDH is often not recognized immediately. It can cause problems such as headache, dizziness, seizure, and hemiparesis, and requires surgical drainage. Most patients with CSDH can be treated safely but may have detrimental sequelae. Mori et al.11) reported severe complications of surgical drainage of postoperative CSDH, such as tension pneumocephalus and acute subdural hematoma, which can cause permanent neurologic deficits or death.
Currently, the incidence and risk factors of postoperative CSDH remain unclear. The general incidence of CSDH after brain surgery is reported to be approximately 0.3-1.5%8,12) however, aneurysm clipping surgery is regarded as an important risk factor of postoperative CSDH7,10,11). It is known that advanced age (>70 years) and male gender may contribute to CSDH after clipping surgery5,12). Additionally, postclipping CSDH is associated with unruptured aneurysm in previous study5). Considering the relatively benign course of unruptured aneurysms, it is important to prevent CSDH and identify its risk factors after clipping surgery in order to minimize treatment-related morbidity. The purpose of this study was to determine the incidence and risk factors of postoperative CSDH after surgical clipping for unruptured anterior circulation aneurysms.
Between January 2008 to December 2013, 980 consecutive patients with unruptured aneurysm underwent surgical clipping in a single tertiary institute. To reduce technical variables that depend on the surgeon, this study included only patients treated by one senior neurosurgeon. Inclusion criteria were unruptured small to large aneurysms (size<2.5 cm) on anterior circulation. Exclusion criteria were giant or complex aneurysms requiring complicated surgical techniques such as bypass surgery. We also excluded distal anterior cerebral artery (ACA) aneurysms and posterior circulation aneurysms that were approached by other than pterional approach, such as anterior frontal craniotomy, subtemporal approach, and etc.
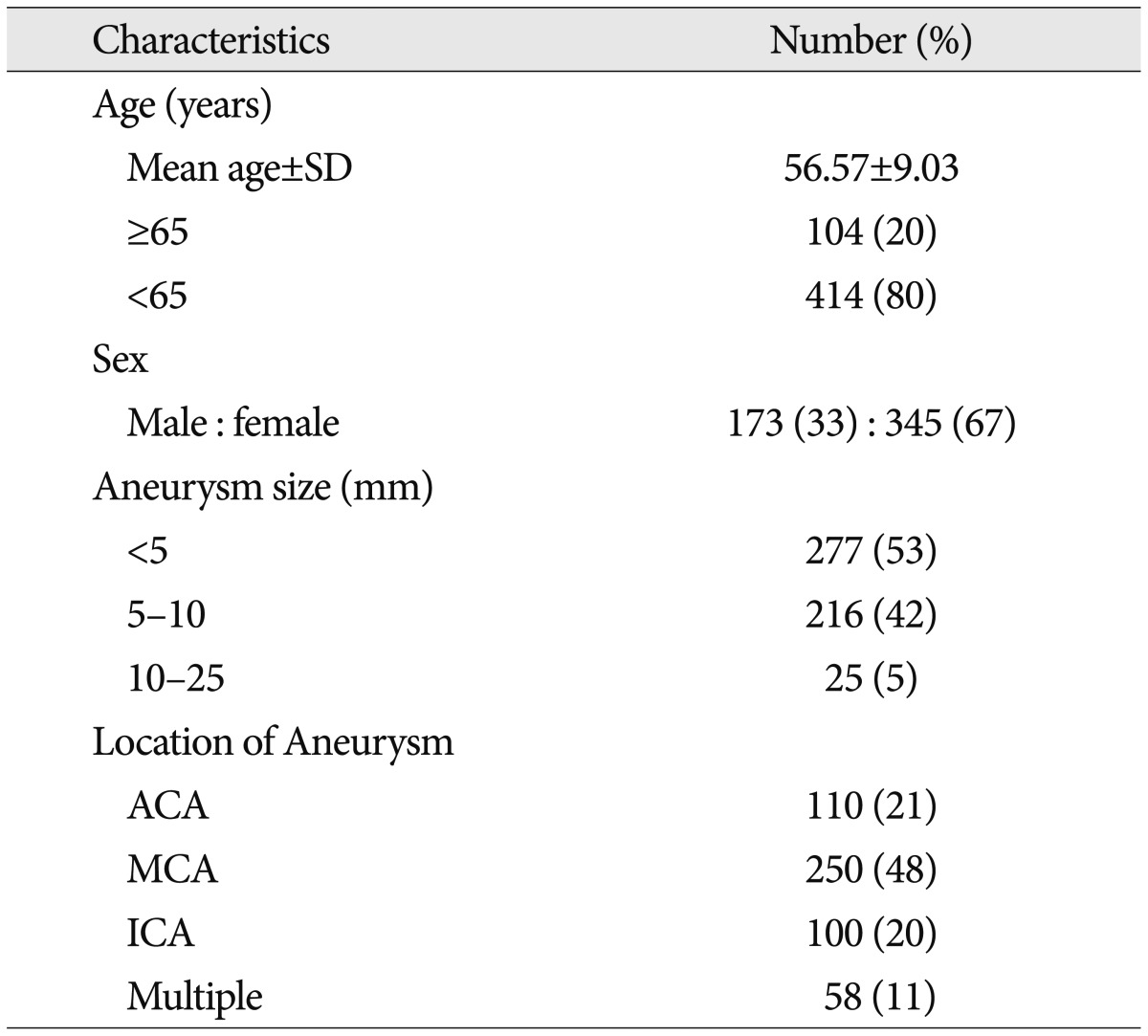
Finally, a total of 518 patients were included in this study. We reviewed their medical records as well as radiological data including age, sex, size and location of aneurysm, size of craniotomy depending on the surgical approach, premature rupture, presence of arachnoid plasty, degree of preoperative brain atrophy, usage of antithrombotics and amount of immediate postoperative subdural fluid collection (SDFC).
We categorized the location of aneurysm as ACA, middle cerebral artery (MCA), and internal carotid artery (ICA) according to the parent artery. ACA included the anterior communicating artery and proximal A2 and A1 aneurysms. ICA included aneurysms from the paraclinoid segment of ICA to ICA bifurcation. MCA aneurysms were defined as aneurysms on the MCA after proximal M1 segment. Aneurysm sizes were divided into three groups based on diameter : tiny (<5 mm), small (5-10 mm), and large (10-25 mm). The size of all aneurysms was its maximal diameter as measured by the three-dimensional reconstructed images of digital subtraction angiography.
The degree of preoperative brain atrophy was estimated using the bicaudate ratio (BCR), which is defined as the ratio of the width of ventricles between the caudate nuclei and internal diameter of the vault at the same level2). BCRs were measured on brain computed tomography (CT) scans that were taken within 6 months prior to surgery. In patients without CT scans, we used magnetic resonance T2-weighted imaging scans that were obtained within same period. BCR indices greater than 0.1 were defined as preoperative brain atrophy2).
Postoperative SDFC was defined as the maximal distance between the inner table of the skull and the cerebral cortex. We measured its maximal vertical depth on the ipsilateral side of the clipped aneurysm on brain CT angiography that was performed 3 or 4 days after surgical clipping. We compared two groups who had postoperative SDFC less than or greater than 10 mm. Finally, postoperative CSDH was defined as symptomatic CSDH that required surgical drainage. Patients had no history of head trauma after clipping surgery. The indication of surgery was determined by patient's symptom. In the case of symptomatic patients with headache or focal neurological deficit, a surgical intervention was performed, whereas in case of asymptomatic patients were treated with conservative management with a clinical follow-up. We excluded the cases of CSDH did not require surgical drainage because we did not perform routine radiological follow-up if they had no progressive symptoms.
After clipping surgery, brain non-contrast CT and intracranial CT angiography was performed on postoperative days 1 and 3 or 4, respectively. Patients were typically discharged 5 to 7 days after surgery. After discharge, clinical follow-ups were routinely performed 1, 3, and 6 months after clipping surgery at our outpatient clinic. Scheduled radiologic follow-ups using intracranial CT angiography were performed after 1 or 2 years. However, imaging studies were employed when any patient showed clinical symptoms or signs that required further evaluation.
Surgical treatments were performed using a standard pterional approach or lateral supraorbital (LSO) approach. The surgical techniques and average craniotomy size of these two approaches have been described previously3,16).
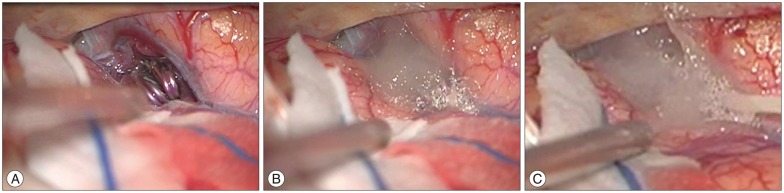
If necessary, temporary clips were placed in order to soften the aneurysm and to reduce intraoperative rupture. Arachnoid plasty has been used since April 2011. The arachnoid plasty technique applied fibrillar [Surgical fibrillar (Ethicon LLC, San Lorenzo, Puerto Rico)] and fibrin glue at the dissected arachnoid surface at the end of surgery (Fig. 1).
Mean and frequency comparisons were performed using Student's t-tests, chi-square (χ2) tests, Mann-Whitney U tests, or Fisher's exact tests as appropriate. Univariate statistical analyses (χ2 tests or Fisher's exact tests) were performed to assess associations between variables and CSDH, the categorical variable. The variables with p-values <0.10 in univariate analyses were selected for multivariate models using multiple logistic regression analyses. Differences were considered significant at p<0.05. Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
The patients enrolled in this study (n=518) consisted of 173 males and 345 females aged 56.57±9.03 years (mean±standard deviation). Table 1 summarizes the baseline characteristics of patients. Of these, 16 (3.1%) patients suffered from postoperative CSDH, which required burr hole drainage surgery.
In univariate analyses, male gender was associated with a higher risk of CSDH (p<0.001). Multivariate analyses also showed similar results [p=0.017, odds ratio (OR) 4.037, range 1.287-12.688]. In this study, we observed no statistically significant correlation with advanced age (≥65 years vs. <65 years, 4.4% vs. 2.7%, p=0.257 based on Fisher's exact test). The factors affecting postoperative CSDH are described in Table 2.
The size of craniotomy does not significantly affect the development of postoperative CSDH. The average maximal vertical depth of immediate postoperative SDFC was 4.42±2.10 mm. The group with higher BCR indices tended to show larger postoperative SDFC in this study (4.0 vs. 4.2, p=0.083 based on Mann-Whitney U test). In addition, the BCR index was associated with postoperative CSDH whereas SDFC was not (Table 2). The location and diameter of aneurysms were positively associated with risk of symptomatic CSDH in univariate analyses. Compared to other locations, ACA aneurysms had higher incidences of CSDH in Fisher's exact test (6 cases, 5.4%, p=0.051). Additionally, larger aneurysms resulted in a higher incidence of CSDH in Fisher's exact test (3 cases, 12%, p=0.030). However, these differences were not significantly different in multivariate analyses using logistic regression test (p=0.231 and p=0.243, respectively). Ninety five of the patients received antithrombotic therapy according to their underlying disease. Of these, 8 patients exhibited CSDH in this study. These findings were statistically significant in uni- and multivariate analyses (p=0.004, OR 4.854, range 1.658-14.085).
All patients who received surgical treatment recovered completely and experienced no complications or permanent neurologic deficits. No patient had recurrence of CSDH, and complete CSDH resorption was confirmed by follow-up brain CT scans.
In this study, we found that male gender was associated with CSDH, which agrees with the findings of previous studies5,12). But, the reason of male preponderance can't be explained by the results of this study. Kanat et al.6) suggested that the male preponderance of CSDH in adults may be due to more brain atrophy and increased in cerebrospinal fluid (CSF) in male gender and estrogens may have a protective effect on the capillaries. Brain atrophy was also associated with postoperative CSDH in this study, but other factors such as increment of CSF or hormonal effect should be considered again in future study. In addition, previous studies have demonstrated that postoperative SDFC and advanced age were associated with postoperative CSDH5,12,15). We hypothesized that the degree of preoperative brain atrophy estimated by BCR and the degree of postoperative SDFC affect the development of postoperative CSDH17). Postoperative SDFC can result from a newly developed artificial CSF tract from the torn arachnoid membrane to the subdural space created by surgical dissection9). If a patient has a higher degree of brain atrophy, the maximal depth of postoperative SDFC could also be higher. CSDH tends to occur in elderly people because brain atrophy causes enlargement of the subarachnoid space and stretching of the bridging veins. These pre-existing conditions facilitate tearing of the arachnoid membrane and leakage of bloody CSF into the subdural space after a neurosurgical procedure, thus producing overdrainage of CSF from the subarachnoid space or the ventricular system13). A previous study demonstrated that SDFC after aneurysm clipping might contribute to CSDH12). In theory, these explanations appear to be acceptable. Our data indicate that higher BCR indices tended to show larger SDFC. However, SDFC itself was not associated with CSDH. These differences can be explained by the following : first, the maximal vertical depth of the SDFC did not represent its total volume. If the exact volume of SDFC could be measured, the results might be different. Second, the timing of brain CT scans differed and we did not checked serial follow-up CT scans, which can determine whether SDFC increased. Changes in SDFC might be more predictive of the incidence of CSDH rather a single measurement of SDFC. In this study, the degree of brain atrophy using BCR is a better predictor than age or SDFC to predict postoperative CSDH.
In order to prevent CSF leakage from the subarachnoid space to the subdural space, arachnoid plasty was performed to seal the opened subarachnoid space. Yet the arachnoid plasty is not a generally accepted procedure in unruptured aneurysm surgery, but sealing of dissected arachnoid plane as an effort to prevent CSF leakage seems to be convincing. If SDFC after craniotomy was related to CSDH occurrence, effective arachnoid plasty would reduce postoperative CSDH. Mino et al.9) reported that arachnoid plasty using fibrin glue prevented SDFC after ruptured aneurysm surgery. So we conducted arachnoid plasty in unruptured aneurysm surgery. Unfortunately, in this study, their procedure did not work as expected. Differences between the Mino et al.9) study and our study included aneurysm status (ruptured vs. unruptured) and number of patients. Moreover, although this technique is simple, technical differences can exist. The current data suggest that arachnoid plasty is ineffective in unruptured aneurysm surgery. Additionally, fibrin glue could spontaneously deteriorate over time, thus resulting in continuous CSF leakage. However, the number of patients included in the previous study was relatively small; therefore, it is difficult to conclude that arachnoid plasty has significant general efficacy. Further studies are required to determine the detailed technical procedure as well as the role of arachnoid plasty in aneurysm surgery.
The reported incidence of postoperative CSDH after brain tumor removal is 0.4%, and the incidence after aneurysm clipping surgery is 2.4%12). The main difference between these types of surgery is the extent of arachnoid dissection. In this study, ACA location tended to be associated with CSDH in univariate analyses and may have correlated with the range of cisternal dissection to soften the brain and expose the aneurysm. ACA aneurysm surgery usually requires dissection of the sylvian fissure, chiasmatic cistern, and interhemispheric cistern; however, other procedures require less. These procedures create a new CSF tract from the subarachnoid space to the subdural space4). Although immediate SDFC was not associated with the incidence of postoperative CSDH, considering the higher incidence of CSDH in clipping surgery, perhaps the continuous leakage of CSF through the surgically-dissected cisternal space may contribute to delayed CSDH that requires surgical intervention. The maximal depth and amount of immediate postoperative SDFC can be larger in mass debulking surgeries, such as brain tumor removal, compared to aneurysm clipping. Indeed, most subdural fluid collections are asymptomatic and disappear spontaneously and have thus not received much attention15,17). Authors thought that the association of ACA aneurysms with CSDH could suggest that more extensive cisternal dissection would have a clinical role in the development of postoperative CSDH.
In our data set, we observed a strong association between CSDH and the use of antithrombotic medication. In our perioperative antithrombotic management strategy, we stopped medications such as aspirin or clopidogrel 7 days before surgery. In patients using antithrombotics, we corrected the bleeding tendency just before surgery. Antithrombotic therapy was started again after we confirmed there was no evidence of hemorrhage on brain non-contrast CT scans 1 day post-surgery. The use of antithrombotic medication is known as an independent risk factor for CSDH in general1,14). It also associates with postoperative CSDH. Therefore, patients using antithrombotic medication require special attention due to the potential risk of CSDH. Also optimal timing of resuming antithrombotics to reduce postoperative CSDH should be identified in further study.
This study has several limitations. First, this study was retrospective in nature. We did not set routine radiological follow-up protocol for observing natural changes of asymptomatic postoperative CSDH. Second, the history of coexisting medical diseases was not fully considered, and could increase the risk of bleeding. Prospective studies are necessary to further elucidate the incidence and risk factors of postoperative CSDH after surgical clipping.
The incidence of CSDH was 3.1% after unruptured anterior circulation aneurysm surgery. This study shows that male gender, degree of brain atrophy, and the use of antithrombotic medication were associated with postoperative CSDH. However, the role of arachnoid plasty in unruptured aneurysm surgery remains unclear.
References
1. Aspegren OP, Åstrand R, Lundgren MI, Romner B. Anticoagulation therapy a risk factor for the development of chronic subdural hematoma. Clin Neurol Neurosurg. 2013; 115:981–984. PMID: 23128014.

2. Barr AN, Heinze WJ, Dobben GD, Valvassori GE, Sugar O. Bicaudate index in computerized tomography of Huntington disease and cerebral atrophy. Neurology. 1978; 28:1196–1200. PMID: 152416.

3. Cha KC, Hong SC, Kim JS. Comparison between lateral supraorbital approach and pterional approach in the surgical treatment of unruptured intracranial aneurysms. J Korean Neurosurg Soc. 2012; 51:334–337. PMID: 22949961.

4. Eguchi S, Aihara Y, Hori T, Okada Y. Postoperative extra-axial cerebrospinal fluid collection--its pathophysiology and clinical management. Pediatr Neurosurg. 2011; 47:125–132. PMID: 21893956.

5. Inamasu J, Watabe T, Ganaha T, Yamada Y, Nakae S, Ohmi T, et al. Clinical characteristics and risk factors of chronic subdural haematoma associated with clipping of unruptured cerebral aneurysms. J Clin Neurosci. 2013; 20:1095–1098. PMID: 23669172.

6. Kanat A, Kayaci S, Yazar U, Kazdal H, Terzi Y. Chronic subdural hematoma in adults : why does it occur more often in males than females? Influence of patient's sexual gender on occurrence. J Neurosurg Sci. 2010; 54:99–103. PMID: 21423076.
7. Koizumi H, Fukamachi A, Nukui H. Postoperative subdural fluid collections in neurosurgery. Surg Neurol. 1987; 27:147–153. PMID: 3810442.

8. Komatsu S, Takaku A, Hori S. [Three cases of chronic subdural hematoma developing after direct aneurysmal surgery (author's transl)]. No Shinkei Geka. 1977; 5:1273–1277. PMID: 593519.
9. Mino Y, Hirashima Y, Hamada H, Masuoka T, Yamatani K, Takeda S, et al. Effect of arachnoid plasty using fibrin glue membrane after clipping of ruptured aneurysm on the occurrence of complications and outcome in the elderly patients. Acta Neurochir (Wien). 2006; 148:627–631. discussion 631. PMID: 16763872.

10. Mori K, Maeda M. Risk factors for the occurrence of chronic subdural haematomas after neurosurgical procedures. Acta Neurochir (Wien). 2003; 145:533–539. discussion 539-540. PMID: 12910395.

11. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases : clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001; 41:371–381. PMID: 11561347.

12. Ohno T, Iihara K, Takahashi JC, Nakajima N, Satow T, Hishikawa T, et al. Incidence and risk factors of chronic subdural hematoma after aneurysmal clipping. World Neurosurg. 2013; 80:534–537. PMID: 23072878.

13. Quintana LM. Chronic subdural hematoma after neurosurgical procedures. World Neurosurg. 2013; 80:482–483. PMID: 23159423.

14. Sim YW, Min KS, Lee MS, Kim YG, Kim DH. Recent changes in risk factors of chronic subdural hematoma. J Korean Neurosurg Soc. 2012; 52:234–239. PMID: 23115667.

15. Takahashi Y, Ohkura A, Sugita Y, Sugita S, Miyagi J, Shigemori M. Postoperative chronic subdural hematoma following craniotomy--four case reports. Neurol Med Chir (Tokyo). 1995; 35:78–81. PMID: 7753312.
16. Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975; 3:7–14. PMID: 1111150.
17. Yoshimoto T, Houkin K, Ishikawa T, Abe H. Arachnoid membrane closure. Prevention of postoperative cerebrospinal fluid leakage. Surg Neurol. 1999; 52:68–71. discussion 71-72. PMID: 10390177.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download