Abstract
Pigmented villonodular synovitis (PVNS) is a benign proliferative joint disease with an uncertain etiology that uncommonly involves the spine. We present a case of PVNS involving the lumbar spine. A 38-year-old male developed back pain and pain in both legs caused by a mass in the L4 region of the right lamina. After gross total tumor removal, the symptoms improved. The pathological finding was synovial hyperplasia with accumulation of hemosiderin-laden macrophages. He was diagnosed with PVNS and experienced no recurrence for up to 2 years after surgery. In this report, we review the previous literature and discuss etiology, clinical manifestations, diagnosis, and treatment.
Pigmented villonodular synovitis (PVNS) is a benign proliferative disease of the synovial membrane with an uncertain etiology. This tumor is characterized by the presence of inflammation and hemosiderin deposition in the synovium. PVNS commonly involves the large appendicular joints, but the axial skeletal system is rarely involved20,22,34,44,47). After the first reported case of PVNS involving the spine at 198029), only a few cases have been reported in the English-language literature. The etiology, natural history, and treatment of PVNS remain unclear. We present a case of PVNS and review the pertinent literature.
A 38-year-old man had complained of an 8-month history of lower back pain and pain in both legs. His symptoms became particularly apparent after an exercise session one month prior to presentation when he noticed that claudication developed after a long walk. He had no antecedent trauma history. An initial neurological examination demonstrated full and symmetric muscle strength in the lower extremities. Sensation was intact to all modalities, and reflexes were symmetrical and nonpathological.
Plain radiographs of the lumbar spine revealed spondylolisthesis at L4-5. Magnetic resonance imaging (MRI) revealed a well-circumscribed 1.4-cm soft tissue mass in the region of the right lamina of L4 (Fig. 1, 2). This lesion originated from the right superior articular process of the L4 vertebra with extension to the spinal canal, forming an extradural mass that compressed the thecal sac.
A midline incision and L3-4 partial hemilaminectomy with a right approach was performed to resect the mass. The ligamentum flavum was thickened with inflammatory changes. After removing the ligamentum flavum, a yellowish-dark colored mass attached to the thecal sac was noted. Gross total removal was performed without any damage to the surrounding tissues.
A histopathologic examination revealed a tumor composed of thick-walled blood vessels with extensive fibrosis and inflamed granulation tissue, and synovial hyperplasia with accumulation of hemosiderin-laden macrophages (Fig. 3). A diagnosis of PVNS was made.
The patient tolerated surgery well and made an uneventful recovery. The pain in the lower back and both legs improved significantly immediately after surgery, although there was some residual pain in the left calf. Follow-up contrast enhanced MRI per-formed 1 month after surgery revealed the absence of any residual mass (Fig. 4). No recurrence of symptoms was observed over the next 2 years.
The World Health Organization classification defines diffuse PVNS as a diffuse-type giant cell tumor that differentiates from a giant cell tumor of the tendon sheath and localized PVNS19). The nomenclature that describes a synovial-type giant cell tumor reflects the uncertain pathogenesis and various clinical manifestations : tenosynovitis, diffuse type PVNS, xanthomatous giant cell tumor, histiocytic giant cell tumor, xanthoma, benign synovialoma, hemorrhagic villous arthritis, and localized or diffuse pigmented villonodular synovitis. Among them, most synovial-type giant cell tumors of the spine published to date have been called pigmented villonodular synositis.
Furlong et al.20) reported 15 cases of a synovial-type giant cell tumor of the vertebral column. Eight cases displayed a diffuse growth pattern, and only one showed typical PVNS morphology. One case of the remaining seven cases was a localized pattern and six cases were an intermediate growth pattern that was difficult to subclassify. Most reports of synovial-type giant cell tumors have excluded a description of the pathological subtype, perhaps because the pathological subtype of PVNS is not related to the surgical plan for spinal PVNS cases, unlike cases involving the appendicular joints. Appendicular tenosynovial giant cell tumors are typically a diffuse type that displays a higher recurrence rate than that of the localized type. However, the small number of reported cases involving the spine and lack of information on subtype hinders typing.
The etiology of PVNS remains controversial. To date, hyperplasia, local metabolic disturbance, recurrent hemorrhage, and trauma have been mentioned as possible etiologic factors, while the inflammatory reaction and neoplasm are less clear and contentious2,21,23,50). The reason for the confusion concerning the causative factor of inflammation is that the cells involved are usually inflammatory cells, such as polyclonal CD8 positive T cells32,37), whereas the presence of trisomy 7 and clonal DNA rearrangements represent the neoplasm1,3,41).
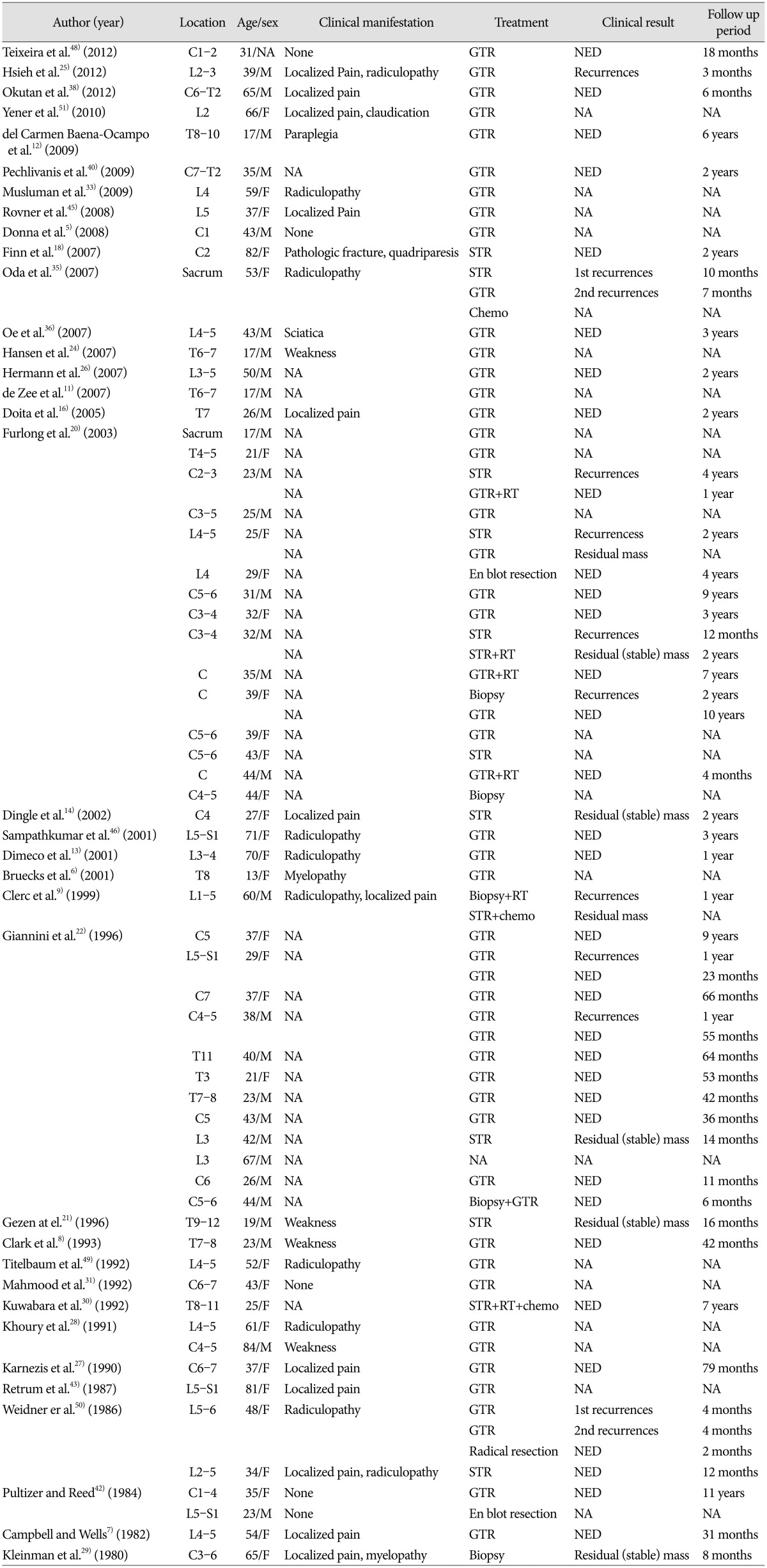
We reviewed 63 cases of spinal PVNS reported in the English literature (Table 1). Median patient age was 37 years (range, 13-84 years), and males were younger (35 years) than females (39 years). Twenty-eight cases (44.4%) were identified in the cervical spine, 12 cases (19%) in the thoracic spine and 21 cases (33.3%) in the lumbar spine. As the lesion origin facet joint mostly contains synovium, spinal PVNS is typically located in the posterior element20). Rare cases have been reported in which spinal PVNS arose from the atlantoaxial or atlantooccipital joints, which are a tendon sheath or synovial membrane containing joints, and not a facet joint5,18,20). Additionally, spinal PVNS involving anterior spinal structures has been reported22,42). It is thought to arise from synovial membranes of the vertebral column accessory joints. Pain localized to the spinal region is the most common symptom. As PVNS tends to arise from the posterior elements, it is often accompanied by radicular pain or myelopathic deficits6,20).
The pathological findings of PVNS in the appendicular skeleton and spine are similar28). However, PVNS of the spine often exhibits less pigmentation than that in the appendicular joint39). The histological appearance is characterized by a proliferation of synovial cells and includes lipid and hemosiderin-laden macrophages, multinucleated giant cells, mixed round cell infiltration, mononuclear polyhedral cells of fibrohistiocytic origin, and stromal and fibroblast cell proliferation4,10). Diffuse-type PVNS has greater vascularity, a greater number of hemosiderin-laden macrophages, and a typically villous pattern, which is often absent in localized PVNS34).
The best choice of diagnosis for PVNS is magnetic resonance imaging (MRI). MRI shows the mass as mixed signal intensity on T2-weighted images due to hemosiderin deposition and can reveal the extent of the lesion, bony erosion, and invasion. Differential diagnoses includes primary bone lesion, mesenchymal neoplasm and extradural masses, such as osteoblastoma, fibrohistiocytic tumor, schwannoma, hemangiopericytoma, tendinous xanthoma, and hypertrophic synovitis22).
The treatment goal for patients with PVNS is gross total excision of the mass with functional preservation and lowering of recurrence rate20). The local recurrence rate of spinal PVNS is 18-25% for all spinal cases22). It is similar with the rate of appendicular PVNS reported in different studies (0-50%)14,17,23,34). Various treatments for PVNS include surgery, radiation therapy, radioisotope infusions, and chemotherapy8,21,22,33,48). Surgical excision with the aim of gross total resection is accepted as the treatment of choice. However, after surgery, evidence is lacking concerning the need for adjuvant treatment to lower the recurrence rate as well as the standard protocol for recurrent PVNS. The extent of resection is currently the only known influence on recurrence rate20,22). Among the 63 cases we reviewed, only four of 46 patients (8.7%) who underwent gross total resection recurred, whereas four of 10 patients (40%) who underwent subtotal resection recurred (Table 1). Three patients received adjuvant chemotherapy and six patients received radiotherapy (RT). No recurrence was found for patients who received adjuvant chemotherapy, but one patient who received RT recurred. The efficacy of the treatment is unclear because there were an insufficient number of patients who received chemotherapy or RT. Therefore, surgical resection remains the treatment of choice for PVNS, unless chemotherapy or RT is a treatment option.
PVNS involving the spine is rare. The recommended method for treating spinal PVNS is gross total removal. No consensus has been reached regarding the efficacy of chemotherapy or RT as adjuvant treatment, because only a few cases have been presented. Therefore, surgical resection should be considered the first treatment for PVNS. More case studies of spinal PVNS are needed to clarify treatment following surgery.
References
1. Abdul-Karim FW, el-Naggar AK, Joyce MJ, Makley JT, Carter JR. Diffuse and localized tenosynovial giant cell tumor and pigmented villonodular synovitis : a clinicopathologic and flow cytometric DNA analysis. Hum Pathol. 1992; 23:729–735. PMID: 1319390.

2. Bentley G, McAuliffe T. Pigmented villonodular synovitis. Ann Rheum Dis. 1990; 49:210–211. PMID: 2187416.

3. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997; 21:153–163. PMID: 9042281.

4. Bisbinas I, De Silva U, Grimer RJ. Pigmented villonodular synovitis of the foot and ankle : a 12-year experience from a tertiary orthopedic Oncology Unit. J Foot Ankle Surg. 2004; 43:407–411. PMID: 15605054.

5. Blankenbaker DG, Tuite MJ, Koplin SA, Salamat MS, Hafez R. Tenosynovial giant cell tumor of the posterior arch of C1. Skeletal Radiol. 2008; 37:667–671. PMID: 18309482.

6. Bruecks AK, Macaulay RJ, Tong KA, Goplen G. November 2000 : 13 year old girl with back pain and leg weakness. Brain Pathol. 2001; 11:263–264. PMID: 11303802.
7. Campbell AJ, Wells IP. Pigmented villonodular synovitis of a lumbar vertebral facet joint. J Bone Joint Surg Am. 1982; 64:145–146. PMID: 7054199.

8. Clark LJ, McCormick PW, Domenico DR, Savory L. Pigmented villonodular synovitis of the spine. Case report. J Neurosurg. 1993; 79:456–459. PMID: 8360747.
9. Clerc D, Berge E, Benichou O, Paule B, Quillard J, Bisson M. An unusual case of pigmented villonodular synovitis of the spine : benign aggressive and/or malignant? Rheumatology (Oxford). 1999; 38:476–477. PMID: 10371292.

10. Darling JM, Goldring SR, Harada Y, Handel ML, Glowacki J, Gravallese EM. Multinucleated cells in pigmented villonodular synovitis and giant cell tumor of tendon sheath express features of osteoclasts. Am J Pathol. 1997; 150:1383–1393. PMID: 9094994.
11. de Zee M, Hansen L, Wong C, Rasmussen J, Simonsen EB. A generic detailed rigid-body lumbar spine model. J Biomech. 2007; 40:1219–1227. PMID: 16901492.

12. del Carmen Baena-Ocampo L, Rosales Olivares LM, Arriaga NM, Izaguirre A, Pineda C. Pigmented villonodular synovitis of thoracic facet joint presenting as rapidly progressive paraplegia. J Clin Rheumatol. 2009; 15:393–395. PMID: 19955996.

13. Dimeco F, Rizzo P, Li KW, Ciceri E, Casali C, Pollo B, et al. Pigment villonodular synovitis of the spine Case report and review of the literature. J Neurosurg Sci. 2001; 45:216–219. discussion 219. PMID: 11912473.
Dingle SR., Flynn JC., Flynn JC Jr., Stewart G. Giant-cell tumor of the tendon sheath involving the cervical spine. A case report. J Bone Joint Surg Am. 2002. 84-A:1664–1667. PMID: 12208926.
15. Docken WP. Pigmented villonodular synovitis : a review with illustrative case reports. Semin Arthritis Rheum. 1979; 9:1–22. PMID: 115092.

16. Doita M, Miyamoto H, Nishida K, Nabeshima Y, Yoshiya S, Kurosaka M. Giant-cell tumor of the tendon sheath involving the thoracic spine. J Spinal Disord Tech. 2005; 18:445–448. PMID: 16189458.

17. Dorwart RH, Genant HK, Johnston WH, Morris JM. Pigmented villonodular synovitis of synovial joints : clinical, pathologic, and radiologic features. AJR Am J Roentgenol. 1984; 143:877–885. PMID: 6332499.

18. Finn MA, McCall TD, Schmidt MH. Pigmented villonodular synovitis associated with pathological fracture of the odontoid and atlantoaxial instability. Case report and review of the literature. J Neurosurg Spine. 2007; 7:248–253. PMID: 17688068.

19. Fletcher CDM, Unni KK, Mertens F. World Health Organization. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press;2002.
20. Furlong MA, Motamedi K, Laskin WB, Vinh TN, Murphey M, Sweet DE, et al. Synovial-type giant cell tumors of the vertebral column : a clinicopathologic study of 15 cases, with a review of the literature and discussion of the differential diagnosis. Hum Pathol. 2003; 34:670–679. PMID: 12874763.

21. Gezen F, Akay KM, Aksu AY, Bedük A, Seber N. Spinal pigmented villonodular synovitis : a case report. Spine (Phila Pa 1976). 1996; 21:642–645. PMID: 8852323.
22. Giannini C, Scheithauer BW, Wenger DE, Unni KK. Pigmented villonodular synovitis of the spine : a clinical, radiological, and morphological study of 12 cases. J Neurosurg. 1996; 84:592–597. PMID: 8613850.

23. Granowitz SP, D'Antonio J, Mankin HL. The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin Orthop Relat Res. 1976; (114):335–351. PMID: 770040.
24. Hansen MA, Harper C, Yiannikas C, McGee-Collett M. A rare presentation of pigmented villonodular synovitis. J Clin Neurosci. 2007; 14:386–388. PMID: 17240150.

25. Hsieh YC, Chen WY, Hsieh TY, Chan WP. Pigmented villonodular synovitis of the lumbar spine. J Clin Rheumatol. 2012; 18:274–275. PMID: 22832299.

26. Hermann J, Stadlmaier E, Aigner Ch, Spuller E, Reittner P, Graninger W. [Erosive intervertebral joint lesions A case of pigmented villonodular synovitis]. Z Rheumatol. 2007; 66:152154–156. PMID: 16988846.
27. Karnezis TA, McMillan RD, Ciric I. Pigmented villonodular synovitis in a vertebra. A case report. J Bone Joint Surg Am. 1990; 72:927–930. PMID: 2365726.

28. Khoury GM, Shimkin PM, Kleinman GM, Mastroianni PP, Nijensohn DE. Computed tomography and magnetic resonance imaging findings of pigmented villonodular synovitis of the spine. Spine (Phila Pa 1976). 1991; 16:1236–1237. PMID: 1754944.

29. Kleinman GM, Dagi TF, Poletti CE. Villonodular synovitis in the spinal canal : case report. J Neurosurg. 1980; 52:846–848. PMID: 7381545.
30. Kuwabara H, Uda H, Nakashima H. Pigmented villonodular synovitis (giant cell tumor of the synovium) occurring in the vertebral column. Report of a case. Acta Pathol Jpn. 1992; 42:69–74. PMID: 1557991.

31. Mahmood A, Caccamo DV, Morgan JK. Tenosynovial giant-cell tumor of the cervical spine. Case report. J Neurosurg. 1992; 77:952–955. PMID: 1331365.
32. Mertens F, Jin Y, Heim S, Mandahl N, Jonsson N, Mertens O, et al. Clonal structural chromosome aberrations in nonneoplastic cells of the skin and upper aerodigestive tract. Genes Chromosomes Cancer. 1992; 4:235–240. PMID: 1382565.

33. Müslüman AM, Cavuşoğlu H, Yilmaz A, Dalkiliç T, Tanik C, Aydin Y. Pigmented villonodular synovitis of a lumbar intervertebral facet joint. Spine J. 2009; 9:e6–e9. PMID: 19303820.

34. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis : a clinical epidemiologic study of 166 cases and literature review. Medicine (Baltimore). 1980; 59:223–238. PMID: 7412554.
35. Oda Y, Takahira T, Yokoyama R, Tsuneyoshi M. Diffuse-type giant cell tumor/pigmented villonodular synovitis arising in the sacrum : malignant form. Pathol Int. 2007; 57:627–631. PMID: 17685937.

36. Oe K, Sasai K, Yoshida Y, Ohnari H, Iida H, Sakaida N, et al. Pigmented villonodular synovitis originating from the lumbar facet joint : a case report. Eur Spine J. 2007; 16(Suppl 3):301–305. PMID: 17566795.

37. Oehler S, Fassbender HG, Neureiter D, Meyer-Scholten C, Kirchner T, Aigner T. Cell populations involved in pigmented villonodular synovitis of the knee. J Rheumatol. 2000; 27:463–470. PMID: 10685815.
38. Okutan O, Solaroglu I, Ozen O, Saygili B, Beskonakli E. Tenosynovial giant cell tumor in the cervico-thoracic junction. Turk Neurosurg. 2012; 22:769–771. PMID: 23208911.

39. Parmar HA, Sitoh YY, Tan KK, Teo J, Ibet S M, Hui F. MR imaging features of pigmented villonodular synovitis of the cervical spine. AJNR Am J Neuroradiol. 2004; 25:146–149. PMID: 14729546.
40. Pechlivanis I, Tannapfel A, Tüttenberg J, Harders A, Schmieder K. [Pigmented villonodular synovitis involving the thoracic spine : a case report]. Z Orthop Unfall. 2009; 147:220–224. PMID: 19358079.
41. Perka C, Labs K, Zippel H, Buttgereit F. Localized pigmented villonodular synovitis of the knee joint : neoplasm or reactive granuloma? A review of 18 cases. Rheumatology (Oxford). 2000; 39:172–178. PMID: 10725067.

42. Pulitzer DR, Reed RJ. Localized pigmented villonodular synovitis of the vertebral column. Arch Pathol Lab Med. 1984; 108:228–230. PMID: 6546511.
43. Retrum ER, Schmidlin TM, Taylor WK, Pepe RG. CT myelography of extradural pigmented villonodular synovitis. AJNR Am J Neuroradiol. 1987; 8:727–729. PMID: 3113211.
44. Rochwerger A, Groulier P, Curvale G, Launay F. Pigmented villonodular synovitis of the foot and ankle : a report of eight cases. Foot Ankle Int. 1999; 20:587–590. PMID: 10509687.

45. Rovner J, Yaghoobian A, Gott M, Tindel N. Pigmented villonodular synovitis of the zygoapophyseal joint : a case report. Spine (Phila Pa 1976). 2008; 33:E656–E658. PMID: 18708919.
46. Sampathkumar K, Rajasekhar C, Robson MJ. Pigmented villonodular synovitis of lumbar facet joint : a rare cause of nerve root entrapment. Spine (Phila Pa 1976). 2001; 26:E213–E215. PMID: 11413441.
47. Stevenson JD, Jaiswal A, Gregory JJ, Mangham DC, Cribb G, Cool P. Diffuse pigmented villonodular synovitis (diffuse-type giant cell tumour) of the foot and ankle. Bone Joint J. 2013; 95-B:384–390. PMID: 23450025.

48. Teixeira WG, Lara NA Jr, Narazaki DK, de Oliveira C, Cavalcanti C, Marins LV, et al. Giant-cell tumor of the tendon sheath in the upper cervical spine. J Clin Oncol. 2012; 30:e250–e253. PMID: 22649149.

49. Titelbaum DS, Rhodes CH, Brooks JS, Goldberg HI. Pigmented villonodular synovitis of a lumbar facet joint. AJNR Am J Neuroradiol. 1992; 13:164–166. PMID: 1595436.
50. Weidner N, Challa VR, Bonsib SM, Davis CH Jr, Carrol TJ Jr. Giant cell tumors of synovium (Pigmented villonodular synovitis) involving the vertebral column. Cancer. 1986; 57:2030–2036. PMID: 3955510.

51. Yener U, Konya D, Bozkurt S, Ozgen S. Pigmented villonodular synovitis of the spine : report of a lumbar case. Turk Neurosurg. 2010; 20:251–256. PMID: 20401854.
Fig. 1
T2-weighted sagittal lumbar magnetic resonance image shows a well-circumscribed mass at the L4 epidural space compressing the spinal cord (arrow).
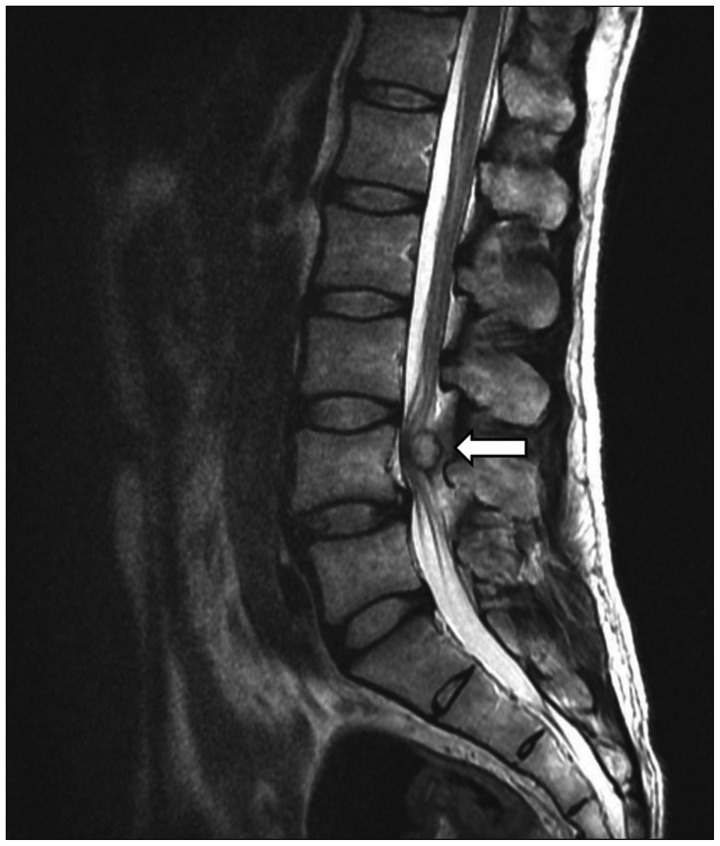
Fig. 2
T2-weighted axial lumbar magnetic resonance image shows a mass originating in the right superior articular process of the L4 vertebrae and extending to the epidural space.
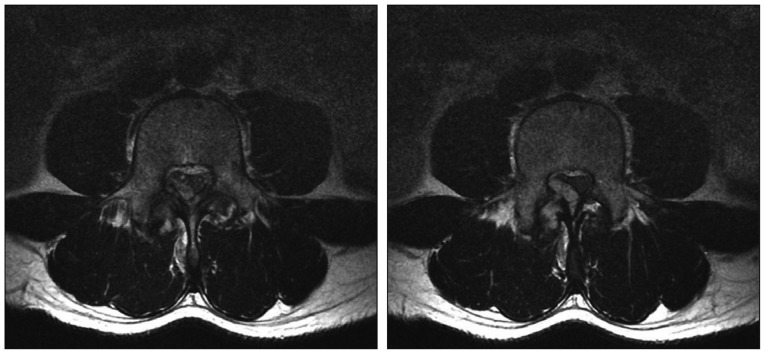
Fig. 3
The surgical specimen shows synovial hyperplasia containing round cells, foamy cells, and hemosiderin-laden macrophages.
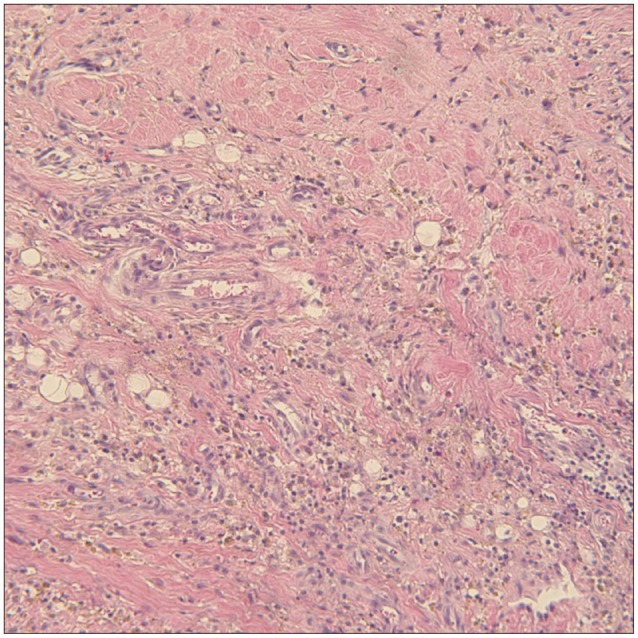
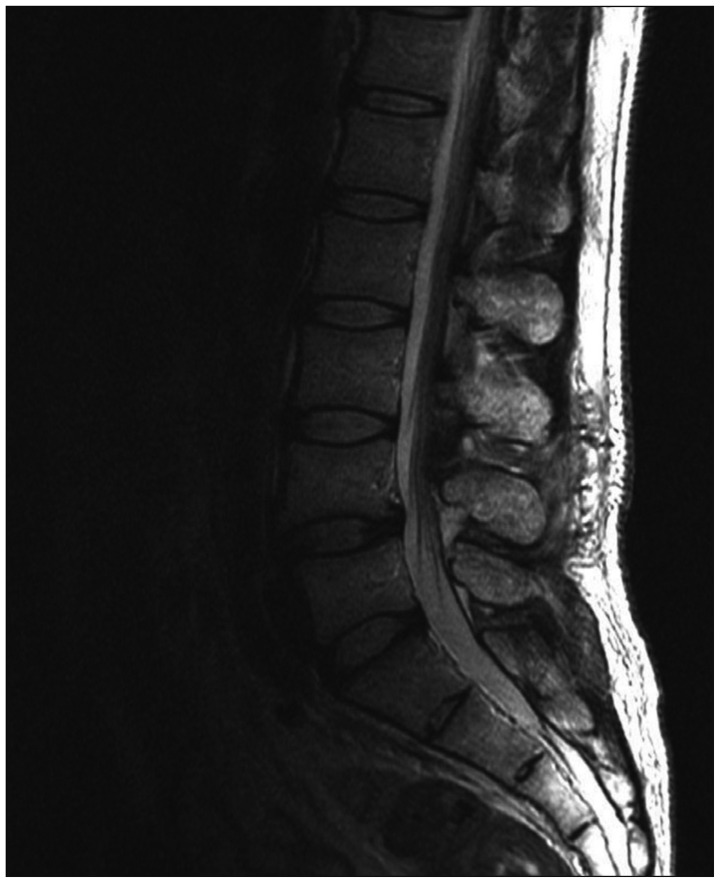
Fig. 4
Postoperative T2-weight saggital lumbar magnetic resonance image shows the absence of the lesion and postoperative changes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download