Abstract
Objective
According to the recent development of minimally invasive spinal surgery, direct lumbar interbody fusion (DLIF) was introduced as an effective option to treat lumbar degenerative diseases. However, comprehensive results of DLIF have not been reported in Korea yet. The object of this study is to summarize radiological and clinical outcomes of our DLIF experience.
Methods
We performed DLIF for 130 patients from May 2011 to June 2013. Among them, 90 patients, who could be followed up for more than 6 months, were analyzed retrospectively. Clinical outcomes were compared using visual analog scale (VAS) score and Oswestry Disability Index (ODI). Bilateral foramen areas, disc height, segmental coronal and sagittal angle, and regional sagittal angle were measured. Additionally, fusion rate was assessed.
Results
A total of 90 patients, 116 levels, were underwent DLIF. The VAS and ODI improved statistically significant after surgery. All the approaches for DLIF were done on the left side. The left and right side foramen area changed from 99.5 mm2 and 102.9 mm2 to 159.2 mm2 and 151.2 mm2 postoperatively (p<0.001). Pre- and postoperative segmental coronal and sagittal angles changed statistically significant from 4.1° and 9.9° to 1.1° and 11.1°. Fusion rates of 6 and 12 months were 60.9% and 87.8%. Complications occurred in 17 patients (18.9%). However, most of the complications were resolved within 2 months.
Direct lumbar interbody fusion (DLIF) using a minimally invasive lateral retroperitoneal transpsoas approach was first reported by McAfee et al.29) Since then, it has been used for the lumbar spine surgery in patients with degenerative spine diseases, trauma or infection.
Minimally invasive transpsoas lumbar interbody fusion has several advantages such as a minimally invasive approach, the increased stability, indirect decompression and the effective correction of coronal balance. Its scope of applications has been extended1,9,21,32). To date, various manufacturers have supplied the surgical equipments and cage. In Korea, however, only the DLIF system (Medtronic, Memphis, TN, USA) is commercially available at present. Accordingly, in Korea, it is the only product that neurosurgeons can use in a clinical setting.
With the expectations for the advantages of DLIF surgery, many study results have been reported worldwide9,24,30,37). It was first introduced in Korea back in May of 2011. Since, its clinical use has been extended. Unfortunately, however, there are no reports about the outcomes of DLIF surgery in Korea. In Korea, the use of medical materials is permitted in a limited scope because of the difference in the medical environment from other countries. It would therefore be mandatory to examine the outcomes of DLIF surgery in Korea, which is essential for determining whether it is appropriate for the body shape and predisposition of Korean people.
Given the above background, we conducted this study to evaluate the clinical and radiological outcomes of DLIF based on our experiences that had been accumulated since the early stage of its introduction in Korea.
Of the 130 patients who underwent DLIF surgery during a period ranging from May of 2011 to December of 2012, 90 could be followed up for more than six months postoperatively and then were enrolled in this retrospective study.
Surgical indications include; 1) spinal stenosis, 2) mild spondylolisthesis of Grade 1 or 2, 3) degenerative scoliosis, and 4) the post-acute phase of infective spondylitis. Moreover, the spinal levels involved T12-L5. The surgical contraindications include; 1) suspected presence of retroperitoneal adhesion due to surgery, 2) severe spondylolisthesis, 3) severe rotational deformity, 4) acute infective spondylitis, 5) L4-5 level with high iliac crest, and 6) L5-S1 level.
Patients were placed in a true lateral position under general endotracheal anesthesia. With the lateral fixation of the bed with the iliac crest placed in the bed corner, the space between the rib and iliac crest is spread. Thus, the space for a surgical approach to the L4-5 lumbar spine can be secured. The posture of the patients and the bed should be adjusted to make sure that the patients should be placed true lateral using a C-arm fluorography. Then, the hip and knee of the patients should be flexed to make sure that the psoas muscle should be relaxed. Using the plaster, the patients were fixed on the bed. After disinfection and draping, the target level was located using a C-arm fluoroscopy. Then, the directions of disc and the sites of skin dissection were marked. At the marked sites, a skin incision was made at a length of approximately 3 cm. This was followed by a blunt dissection of the subcutaneous tissue and three-layer abdominal pad. With the exposure of retroperitoneal fat, the dissection was performed involving the psoas muscle with the use of the index finger. Intraoperative monitoring electrode was placed in the annulus of the center of the disc space through the psoas muscle. Once the safety was assured, the tubular retractor was placed and then fixed there. The exposed space was confirmed using an electrode. After the intervertebral disc was removed, the contralateral annulus was exposed. Again, the endplate preparation was done using both a shaver and a currette. This was followed by the insertion into the disc space in such a condition that the fusion material is placed in a cage with the appropriate size. It would be ideal to use the autologous bone for the bone fusion. Because the DLIF surgery is a minimally invasive modality, it is not easy to harvest the autologous bone from patients. Therefore, the bone substitutes such as the demineralized bone matrix (DBM) or tricalcium phosphate (TCP) are commonly used. In the early stage, we used ChronOS (Synthes, Oberdorf, Switzerland) as the graft material. But it is disadvantageous in that it is loosened during the insertion into the cage. Thereafter, we commonly used Osteofil (Medtronic, Memphis, TN, USA).
In all the patients undergoing DLIF surgery, we performed the posterior fixation using the percutaneous pedicle screw fixation system (SEXTANT II® or Longitude system, Medtronic, Memphis, TN, USA). In addition, we also performed the additional posterior decompression limitedly for patients with severe central spinal stenosis or ruptured disc herniation. In the remaining patients, we performed the indirect decompression only without additional posterior decompression. We performed the DLIF surgery at the spinal levels extending from T12 to L5. We also performed the transforaminal lumbar interbody fusion (TLIF) for patients who were in need of the surgery for the spinal levels of L5-S1.
We compared the clinical outcomes based on the visual analog scale (VAS) scores and Oswestry Disability Index (ODI) between preoperatively and six months postoperatively.
On oblique sagittal computed tomography (CT) scans, showing the greatest area of the foramen, we measured the foramen area on both sides. But we excluded the sites of facetectomy for the additional posterior decompression in measuring the foramen area. To evaluate the preoperative and postoperative radiographs of the lumbar spine, we measured the disc height, segmental coronal, sagittal and regional sagittal angles. Segmental coronal and sagittal angles were measured at the fusion levels. Regional sagittal angle was measured between L1 and S1. Moreover, we measured the cage position based on the center of the cage from the lateral view of the disc space. The anterior, middle and posterior positions were defined as the cage center being placed within the anterior 40% of the vertebral body, between 40% and 60% and >60%, respectively20). We analyzed the postoperative changes in the segmental sagittal angles depending on the cage position.
The fusion was evaluated based on the Bridwell fusion grading system4). According to the Bridwell fusion grading system, the fusion is evaluated as grade 1; completely remodeled with trabeculae across disc space, grade 2; graft intact with no lucent lines seen between graft and adjacent endplates, grade 3; graft intact, but a radiolucent line is seen between the graft and an adjacent endplate, grade 4; lucency along an entire border of the graft, or lucency around a pedicle screw or subsidence of the graft. Based on this classification system, grade 1-2 was determined to be successful fusion. We analyzed the fusion rate at six months postoperatively (n=69) and one year (n=41).
If there was a graft migration to the adjacent endplate at a distance of >2 mm at a 6-month follow-up radiography, the corresponding cases were considered to have a subsidence.
We compared the radiological and clinical outcomes between preoperatively and postoperatively using a paired t-test and Wilcoxon signed-rank test. In addition, we also compared the VAS and ODI on the symptom side using Kruskall-Wallis test. Furthermore, we compared the subsidence using the chi-square test. A p-value of <0.05 was considered statistically significant.
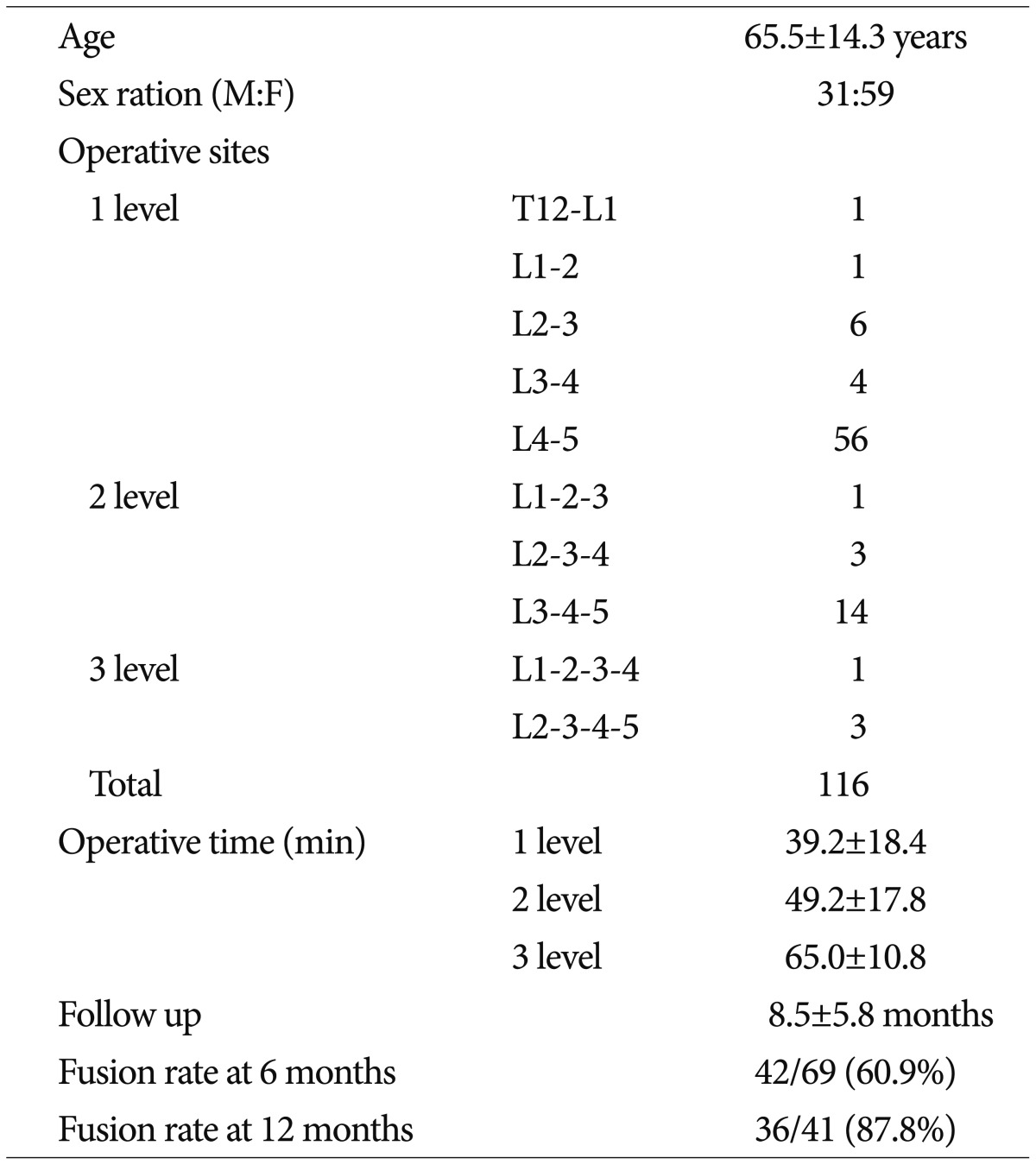
The mean age of the patients was 65.5±14.3 years. Our clinical series of patients comprise 31 men and 59 women. The mean follow-up period was 8.4±5.8 months (range, 6-23 months). In addition, the mean bone mineral density (BMD) T-score was measured as -0.8±1.8 points. The cage width was 18 mm and the lordotic angle was 6°. The cage height was 10 mm in six levels, 12 mm in 53 levels, 14 mm in 53 levels and 16 mm in four levels. The cage length was 45 mm in 12 levels, 50 mm in 54 levels and 55 mm in 50 levels. The surgical sites involved T12-L5; there were 68 cases of one level, 18 cases of two levels and four cases of three levels. Thus, a total of 116 levels were surgically operated. One level includes T12-L1 in one patient, L1-2 in one patient, L2-3 in six patients, L3-4 in four patients and L4-5 in 56 patients. Two levels include L1-2-3 in one patient, L2-3-4 in three patients and L3-4-5 in 14 patients. Three levels include L1-2-3-4 in one patient and L2-3-4-5 in three patients (Table 1). The cage was inserted from the left side in all the cases.
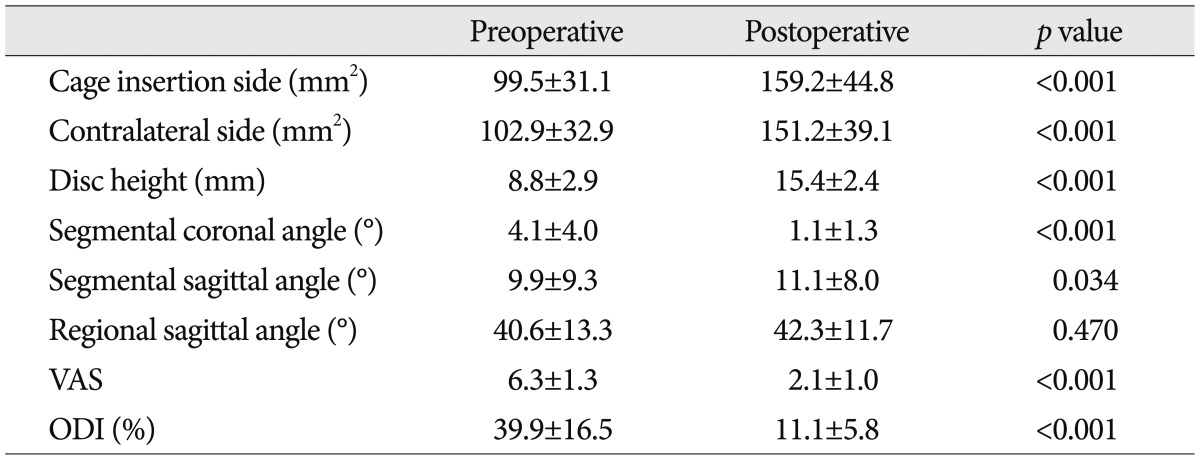
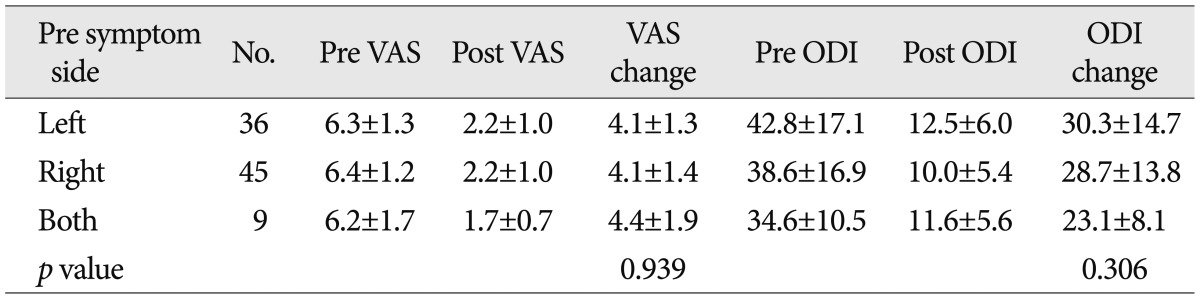
The VAS score and ODI were 6.3±1.7 and 39.9±16.5% preoperatively and 2.1±1.0 and 11.1±5.8% postoperatively (p<0.001) (Table 2). Our clinical series of patients comprise 36 left-sided cases, 45 right-sided cases and nine bilateral cases. But we performed surgery for the left side with no respect to whether the patients presented with symptoms on the specific side. There were no significant differences in the VAS score and ODI between the three groups (Table 3).
The mean operation time of DLIF surgery was 39.2±18.4 min in one-level cases, 49.2±17.8 min in two-level cases and 65.0±10.8 min in three-level cases (Table 1). The amount of blood loss during DLIF surgery could not be measured because it was relatively smaller.
On the left side, the mean foraminal area was 99.5±31.1 mm2 preoperatively and 159.2±44.8 mm2 postoperatively (p<0.001). On the right side, it was 102.9±32.9 mm2 and 151.2±39.1 mm2 in the corresponding order (p<0.001). These results indicate that there was no significant difference in the foraminal area between the left and right side between preoperatively and postoperatively. The disc height was 8.8±2.9 mm preoperatively and 15.4±2.4 mm postoperatively (p<0.001). In addition, the segmental coronal and sagittal angle were 4.1±4.0° and 9.9±9.3° preoperatively and were 1.1±1.3° and 11.1±8.0°, respectively, postoperatively (p<0.001 and p=0.034, respectively). But there was no significant difference in the regional sagittal angle between preoperatively and postoperatively (40.6±13.3° and 42.3±11.7°, respectively) (p=0.470) (Table 2).
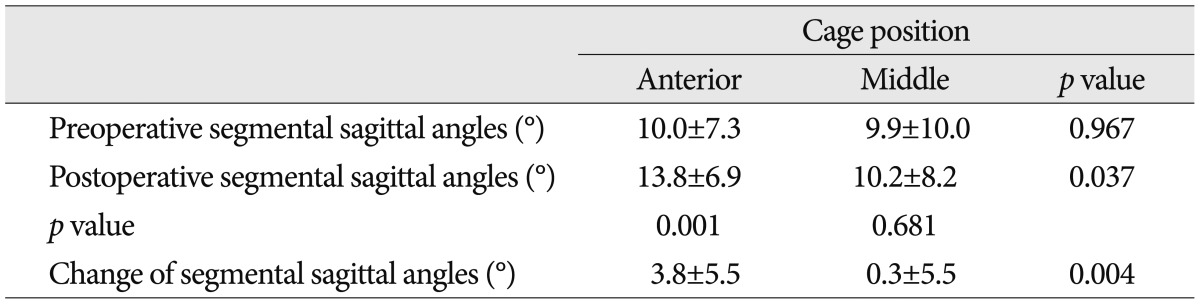
The cage was placed in the anterior position at 32 levels, the middle position at 83 levels and the posterior position at one level. In most of the patients, the cage was placed between the anterior and middle position. In cases in which the cage was placed in the anterior position, the preoperative and postoperative segmental sagittal angles were 10.0±7.3° and 13.8±6.9°, respectively (p=0.001). In cases in which the cage was placed in the middle position, the preoperative and postoperative segmental sagittal angles were 9.9±10.0° and 10.2±8.2°, respectively (p=0.681). The degree of changes in the segmental sagittal angles was 3.8±5.5° in cases in which the cage was placed in the anterior position and 0.3±5.5° in the middle position (p=0.004) (Table 4).
At a 6-month follow-up, 42 of 69 patients had findings that are suggestive of the Bridwell fusion grade 1 or 2. Thus, the fusion rate was 60.9%. In addition, at a 1-year follow-up, 36 of 41 patients had a fusion. Thus, the fusion rate was 87.8% (Table 1).
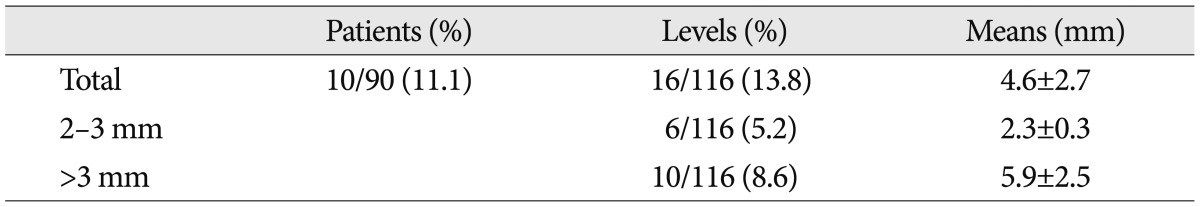
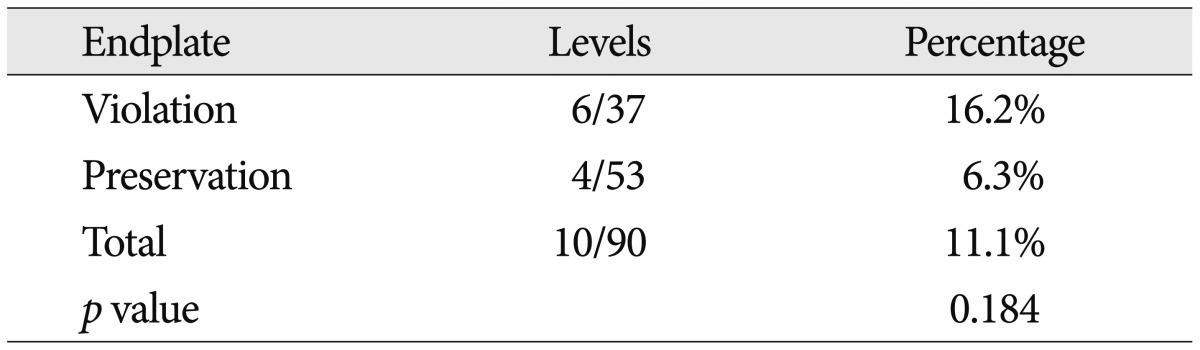
Six months postoperatively, the subsidence was seen at 16 of 115 levels (13.8%) in ten of 90 patients (11.1%). In these 16 levels, the mean degree of subsidence was 4.6±2.7 mm. It was 2-3 mm at six levels (5.2%) and higher than 3 mm at ten levels (8.6%) (Table 5). There were 37 patients who intraoperatively had damage to the endplate. Of these, six patients had a subsidence of >2 mm in degree at a 6-month follow-up. This suggests that there was no significant correlation between the intraoperative damage to the endplate and the postoperative subsidence (p>0.05) (Table 6). In addition, the subsidence had no effects on clinical outcomes such as VAS and ODI (p>0.05).
Complications related with DLIF occurred in 17 patients (18.9%). Eleven patients (12.2%) showed psoas muscle symptoms, 4 patients (4.4%) showed lateral femoral cutaneous nerve symptom, and 2 patients (2.2%) had genitofemoral nerve symptom. However, most of the symptoms were resolved within 2 months. Only one patient with lateral femoral cutaneous nerve symptom complained for 12 months. There was no nerve root or lumbar plexus injury. There were no infections. During the follow-up period, there were no cases for which a revision surgery was needed because of screw loosening, cage migration or pseudoarthrosis.
The DLIF is effective in preserving the anterior longitudinal ligament, the posterior longitudinal ligament and the facet joint. It can therefore well maintain the spinal alignment and stabilization due to the ligamentotaxis. Thus, it is expected to be an alternative surgical modality to conventional various types of lumbar interbody fusion6,11). Moreover, its large cage increases the size of foramen. Thus, it makes the indirect decompression possible. It is also known to have an excellent effect on the coronal and sagittal balance correction1,21).
The DLIF is a more advantageous modality as compared with the anterior lumbar interbody fusion (ALIF), the posterior lumbar interbody fusion (PLIF) and the TLIF. Unlike TLIF or PLIF, DLIF can provide strong mechanical stability by not only using large interbody construct but also sparing ligamentous structures32). From the aspect of the safety, the DLIF is advantageous in achieving the biomechanical stability to the same extent as the ALIF25). As compared with the ALIF, however, the DLIF is advantageous in that it can prevent the occurrence of injury to the abdominal internal organs as well as the peritoneal penetration via a retroperitoneal approach, decrease risks of developing great vessel injury and avoid an injury to the sympathetic chain36). As compared with posterior approaches such as the TLIF and PLIF, the DLIF is advantageous in avoiding the dural tears, nerve root injury and paraspinal muscle injury35).
Parker et al.34) have recently reported that the VAS scores were decreased by approximately 57.3% and the ODI scores were lowered from 35.6% to 19.5% in patients undergoing TLIF. Our results showed that the VAS scores were decreased by approximately 66.7% (from 6.3 to 2.1) and the ODI scores were lowered from 39.9% to 11.1% in patients undergoing DLIF. These results indicate that there were no significant differences in the treatment outcomes between the DILF and the TLIF.
In the current study, we performed surgery at various levels extending from T12 to L5. The DLIF approach can be made the most easily at the L2-3-4 levels because of such structures as the rib cage, the iliac crest and lumbosacral plexus33). At the T12-L1-L2 levels, it is generally recommended that a transthoracic-transdiaphragmatic-retroperitoneal approach be made because the ribs and diaphragm are lowered to the L1-2 levels. In these cases, the length of skin incision can be shortened to smaller than 10 cm as compared with conventional types of transthoracic approach. Moreover, it is not necessary to dissect the ribs for an intercostal approach. Furthermore, it is also advantageous in that it can easily reach the retroperitoneal space although the diaphragm is incised at a small length of approximately 3-4 cm. By contrast, it is difficult to approach the L4-5 level because of such structures as the iliac crest and the lumbosacral plexus33). The iliac crest can be avoided in patients with good position. In most of the patients where the iliac crest cannot be avoided, surgery can be successfully performed via a slightly oblique approach. There is a tendency that the lumbosacral plexus passes the central region of the lateral aspect of the L4-5 disc levels31). The nerve damage can therefore be avoided through an intraoperative monitoring. In our clinical series of patients, approaches to the L4-5 level were made the most prevalently. It can therefore be inferred that the DLIF at this level cannot be avoided. Actually, there was one patient where approaches could not be made because of the iliac crest during the study period. But there were no patients where approaches failed because of the lumbosacral plexus.
According to the report about the size of neural foramen following DLIF, the foraminal area was increased by approximately 35%21). Our results showed that the foramen area was increased from 99.5 mm2 to 159.2 mm2 by 37.5% on the left side where the cage was inserted and from 102.9 mm2 to 151.2 mm2 by 32.0% on the contralateral right side. This is consistent with other reports. Of the two foramen areas, the foramen area was greater on the operated side. But this did not reach a statistical significance. These results indicate that the DLIF might be a very effective modality in indirectly decompressing bilateral foraminal stenosis. Presumably, this might be because the disc height on both sides could be increased both consistently and effectively with the use of a high, long cage.
Johnson et al.19) reported that the segmental lumbar lordosis was significantly increased from 3.0° to 6.6° following DLIF, but there was a non-statistically significant change in the regional lumbar lordosis. Other studies have reported that the DLIF was more effective in forming the segmental lordosis when the cage was anteriorly inserted, thus indicating that the position of cage might affect the lumbar lordosis20). In our series, the cage was inserted in the anterior position at 32 levels, the middle position at 83 levels and the posterior position at one level. Overall, the segmental lordosis was increased from 9.9±9.3° to 11.1±8.0°. This reached a statistical significance (p<0.05). In cases in which the cage was inserted in the anterior position, the segmental sagittal angle was significantly increased from 10.0° to 13.8° (p<0.05). In cases in which the cage was inserted in the middle position, however, it was slightly increased from 9.9° to 10.2° (p>0.05). These results are in agreement with previous reports that the DLIF was effective in forming the lordotic angle when the cage was inserted in the anterior position. Consistent with other studies, there was no significant difference in the regional lumbar lordosis. In our series, we performed surgery at a mean number of levels of 1.3, thus correcting a narrow space between the spinal levels, and this had little effects on total cases of regional lumbar lordosis. Our results also showed, however, that the segmental lordosis was significantly increased; this provides a possibility that the multiple level DLIF might be effective for the treatment of adult deformity2,3,9,18,38).
Acosta et al.1) reported that the coronal segmental angle was corrected from 4.5° preoperatively to 1.5° postoperatively in patients undergoing DLIF. Consistent with previous reports, our results also showed that the segmental coronal angle was corrected from 4.1° to 1.1°. These findings have been consistently reported in studies about the DLIF1,19,38), thus indicating that the DLIF is effective for the correction of coronal deformity.
It is recommended that a substantially long cage be used so that it may be trapped in the apophysis ring during the DLIF. This is because the apophysis is the most powerful structure in the vertebral body and it is useful to reduce the subsidence and to maintain the disc height15,28). Other studies have reported that the graft subsidence occurred in 21.7% of patients undergoing ALIF and 23.5% of those undergoing TLIF17). Our results showed that the subsidence occurred in 11.1% (10/90) of total patients. This suggests that the cage was trapped between the bilateral apophysis rings, thus preventing further occurrence of bone damage. Moreover, our results also showed that there were no further effects on the subsidence even in cases in which the endplate was damaged during surgery. Presumably, this might be because the bilateral apophysis rings tolerates the force.
Le et al.26) reported that the overall subsidence rate was 8.8% in patients undergoing DLIF. According to these authors, however, it was 14.1% in patients undergoing DLIF with a cage of 18 mm in width and then markedly decreased to 1.9% in those undergoing DLIF with a cage of 22 mm in width. Currently in Korea, the cage of 22 mm in width is not commercially available. It is therefore unavoidable that only the cage of 18 mm in width should be used in all the patients. But if there are any chances that the cage of 22 mm in width will be used in Korea, it would markedly lower the current subsidence rate of 11.1%.
The anterior and posterior longitudinal ligaments, both of which are preserved during the DLIF approach, play a role in preventing the anterior and posterior loosening of the cage and enhancing the dynamic stability due to the ligamentotaxis32,33). In our series, there was no anterior and posterior loosening of the cage. Therefore, the above hypothesis can be supported.
According to previous reports about the rate of bone fusion, it was 82.4-93.8% in patients undergoing ALIF, 76.9-97.8% in those undergoing TLIF12,14,22) and 92.6-95.0% in those undergoing PLIF7,10,23). Consistent with these reports, our data showed that the 1-year rate of bone fusion was 87.8%. The DLIF is a minimally-invasive surgery, but it is disadvantageous in that the autologous bone cannot be harvested. Therefore, the bone prosthesis should be mainly used for the DLIF. To overcome this disadvantage, the bone morphogenic protein (BMP) with a high rate of bone fusion is commonly used in overseas countries5,13). In Korea, however, it is not commercially available because it did not obtain the approval from the Korean Food and Drug Administration (KFDA). Accordingly, in Korea, demineralized bone matrix (DBM) is mainly used as a fusion material for the cage during the DLIF. We mainly use the Osteofil (Medtronic, Memphis, TN, USA), a type of DBM, and showed the rate of bone fusion with the use of Osteofil in the current study. Overseas reports have shown that the rate of bone fusion was 89.7% in patients who were treated with both Osteofil and BMP13). To date, however, no Korean authors have reported the rate of bone fusion in patients who were treated solely with DBM. From this point of view, based on our results that the 1-year rate of bone fusion of approximately 87.8% in patients who underwent surgery for the interbody fusion solely using the DBM, the degree of bone fusion would be estimated in patients using the DBM. Of particular note, our results showed that we obtained the successful bone fusion solely using the DBM despite a lack of availability of BMP in patients who could not be treated with the autologous bone graft.
The incidence of complications due to the DLIF varies, ranging from 0.7% to 62.7%8,24,37). Such complications include psoas muscle injury and edema due to a retroperitoneal transpsoas approach, leading to the hip flexor weakness, thigh/groin pain and numbness due to the genitofemoral nerve injury, meralgia paresthetia due to the lateral femoral cutaneous nerve injury and the numbness due to the nerve root injury or lumbosacral plexus injury16,30). With the increased technical expertise, the incidence of complications of minimal invasive transpsoas lateral lumbar interbody fusion operation is gradually decreased to 26.1%, 25%, and 10.7% in surgeons with a 1-, 2-, and 3-year experience, respectively27). Our results also showed that the overall incidence of complications was 18.9% during a follow-up period of one year and six months. But it was 25.9% (15/58) during the first year and 6.3% (2/32) during the following six months. Thus, it was rapidly decreased. This suggests that the DLIF may promptly reduce the complications with the increased technical expertise although it shows a very steep learning curve.
The limitations of the current study are as follows. First, we enrolled a small number of patients. Second, we followed up our clinical series of patients during a short-term period of one and six months. Our results cannot therefore be generalized. In addition, long-term effects of our methods cannot be concluded. Third, we also analyzed the surgical outcomes before achieving a substantial level of technical expertise. Therefore, there is a possibility that the clinical outcomes, imaging study results and complications might vary depending on whether our clinical series of patients underwent surgery in the early or late stage.
Further large-scale, long-term follow-up studies are warranted to overcome the above limitations.
The DLIF may increase the size of both foramen areas, thus achieving an indirect decompression and maintaining the coronal balance. In addition, it showed a stability on imaging studies. Furthermore, it showed a similar rate of bone fusion as compared with other surgical methods and produced a transient onset of a lower incidence of serious complications.
In the early stage, the DLIF shows a slightly steep learning curve. But surgeons can promptly accommodate it. Later on, it might be a safe, effective surgical modality that can be alternatively used to conventional types of interbody fusion surgery. There is an unfavorable situation that the use of cage or fusion material is somewhat limited in Korea. But this had no great impacts on the surgical outcomes.
References
1. Acosta FL, Liu J, Slimack N, Moller D, Fessler R, Koski T. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults : a radiographic study. J Neurosurg Spine. 2011; 15:92–96. PMID: 21476802.

2. Anand N, Baron EM, Thaiyananthan G, Khalsa K, Goldstein TB. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis : a technique and feasibility study. J Spinal Disord Tech. 2008; 21:459–467. PMID: 18836355.

3. Anand N, Rosemann R, Khalsa B, Baron EM. Mid-term to long-term clinical and functional outcomes of minimally invasive correction and fusion for adults with scoliosis. Neurosurg Focus. 2010; 28:E6. PMID: 20192666.

4. Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976). 1995; 20:1410–1418. PMID: 7676341.

5. Burkus JK. Bone morphogenetic proteins in anterior lumbar interbody fusion : old techniques and new technologies. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004; 1:254–260. PMID: 15478362.

6. Cappuccino A, Cornwall GB, Turner AW, Fogel GR, Duong HT, Kim KD, et al. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine (Phila Pa 1976). 2010; 35(26 Suppl):S361–S367. PMID: 21160401.

7. Cheng L, Nie L, Zhang L. Posterior lumbar interbody fusion versus posterolateral fusion in spondylolisthesis : a prospective controlled study in the Han nationality. Int Orthop. 2009; 33:1043–1047. PMID: 18521599.

8. Cummock MD, Vanni S, Levi AD, Yu Y, Wang MY. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine. 2011; 15:11–18. PMID: 21476801.

9. Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010; 28:E8. PMID: 20192668.

10. Dehoux E, Fourati E, Madi K, Reddy B, Segal P. Posterolateral versus interbody fusion in isthmic spondylolisthesis : functional results in 52 cases with a minimum follow-up of 6 years. Acta Orthop Belg. 2004; 70:578–582. PMID: 15669459.
11. Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine (Phila Pa 1976). 2001; 26:E122–E129. PMID: 11246394.

12. Faundez AA, Schwender JD, Safriel Y, Gilbert TJ, Mehbod AA, Denis F, et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration : a retrospective comparative study of 133 patients. Eur Spine J. 2009; 18:203–211. PMID: 19125304.

13. Glassman SD, Carreon L, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, et al. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007; 7:44–49. PMID: 17197332.

14. Hee HT, Castro FP Jr, Majd ME, Holt RT, Myers L. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion : analysis of complications and predictive factors. J Spinal Disord. 2001; 14:533–540. PMID: 11723406.

15. Hollowell JP, Vollmer DG, Wilson CR, Pintar FA, Yoganandan N. Biomechanical analysis of thoracolumbar interbody constructs How important is the endplate? Spine (Phila Pa 1976). 1996; 21:1032–1036. PMID: 8724086.

16. Houten JK, Alexandre LC, Nasser R, Wollowick AL. Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine. 2011; 15:280–284. PMID: 21619401.

17. Hsieh PC, Koski TR, O'Shaughnessy BA, Sugrue P, Salehi S, Ondra S, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion : implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine. 2007; 7:379–386. PMID: 17933310.

18. Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis : perioperative outcomes and complications. Spine (Phila Pa 1976). 2010; 35(26 Suppl):S322–S330. PMID: 21160396.
19. Johnson RD, Valore A, Villaminar A, Comisso M, Balsano M. Pelvic parameters of sagittal balance in extreme lateral interbody fusion for degenerative lumbar disc disease. J Clin Neurosci. 2013; 20:576–581. PMID: 23375396.

20. Kepler CK, Huang RC, Sharma AK, Meredith DS, Metitiri O, Sama AA, et al. Factors influencing segmental lumbar lordosis after lateral transpsoas interbody fusion. Orthop Surg. 2012; 4:71–75. PMID: 22615150.

21. Kepler CK, Sharma AK, Huang RC, Meredith DS, Girardi FP, Cammisa FP Jr, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012; 16:329–333. PMID: 22284229.

22. Kim JS, Kang BU, Lee SH, Jung B, Choi YG, Jeon SH, et al. Mini-transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation : a comparison of surgical outcomes in adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech. 2009; 22:114–121. PMID: 19342933.

23. Kim KT, Lee SH, Lee YH, Bae SC, Suk KS. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine (Phila Pa 1976). 2006; 31:1351–1357. discussion 1358. PMID: 16721298.

24. Knight RQ, Schwaegler P, Hanscom D, Roh J. Direct lateral lumbar interbody fusion for degenerative conditions : early complication profile. J Spinal Disord Tech. 2009; 22:34–37. PMID: 19190432.
25. Laws CJ, Coughlin DG, Lotz JC, Serhan HA, Hu SS. Direct lateral approach to lumbar fusion is a biomechanically equivalent alternative to the anterior approach : an in vitro study. Spine (Phila Pa 1976). 2012; 37:819–825. PMID: 21971125.

26. Le TV, Baaj AA, Dakwar E, Burkett CJ, Murray G, Smith DA, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976). 2012; 37:1268–1273. PMID: 22695245.

27. Le TV, Burkett CJ, Deukmedjian AR, Uribe JS. Postoperative lumbar plexus injury after lumbar retroperitoneal transpsoas minimally invasive lateral interbody fusion. Spine (Phila Pa 1976). 2013; 38:E13–E20. PMID: 23073358.

28. Lowe TG, Hashim S, Wilson LA, O'Brien MF, Smith DA, Diekmann MJ, et al. A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine (Phila Pa 1976). 2004; 29:2389–2394. PMID: 15507800.

29. McAfee PC, Regan JJ, Geis WP, Fedder IL. Minimally invasive anterior retroperitoneal approach to the lumbar spine Emphasis on the lateral BAK. Spine (Phila Pa 1976). 1998; 23:1476–1484. PMID: 9670400.

30. Moller DJ, Slimack NP, Acosta FL Jr, Koski TR, Fessler RG, Liu JC. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg Focus. 2011; 31:E4. PMID: 21961867.

31. Moro T, Kikuchi S, Konno S, Yaginuma H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine (Phila Pa 1976). 2003; 28:423–428. discussion 427-428. PMID: 12616150.

32. Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010; 35(26 Suppl):S331–S337. PMID: 21160397.

33. Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF) : a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006; 6:435–443. PMID: 16825052.

34. Parker SL, Adogwa O, Paul AR, Anderson WN, Aaronson O, Cheng JS, et al. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Neurosurg Spine. 2011; 14:598–604. PMID: 21332281.

35. Potter BK, Freedman BA, Verwiebe EG, Hall JM, Polly DW Jr, Kuklo TR. Transforaminal lumbar interbody fusion : clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech. 2005; 18:337–346. PMID: 16021015.
36. Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999; 91(1 Suppl):60–64. PMID: 10419370.

37. Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion : an analysis of 600 cases. Spine (Phila Pa 1976). 2011; 36:26–32. PMID: 21192221.

38. Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010; 28:E7. PMID: 20192667.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download