Abstract
Glioblastoma multiforme (GBM) is the most common primary brain tumor and the most malignant astrocytoma in adults, with rare extra-cranial metastases, especially for subcutaneous metastases. It could be easily misdiagnosed as primary subcutaneous tumor. In this report, we describe a patient with pontine GBM who developed a subcutaneous swelling at the ipsilateral posterior cervical region 8 months after operation, and the pathological and immunocytochemical examination carry the same characteristics as the primary intracranial GBM cells, which defined it as subcutaneous metastasis. GBM with subcutaneous metastasis is extremely rare, and knowledge of a prior intracranial GBM, pathological examinations and immunocytochemical tests with markers typically expressed by GBM are of vital importance for the diagnosis of GBM metastasis. Surgical resection of subcutaneous swelling, followed by chemotherapy and radiotherapy, could be the best strategy of treatment for the patients with GBM subcutaneous metastasis.
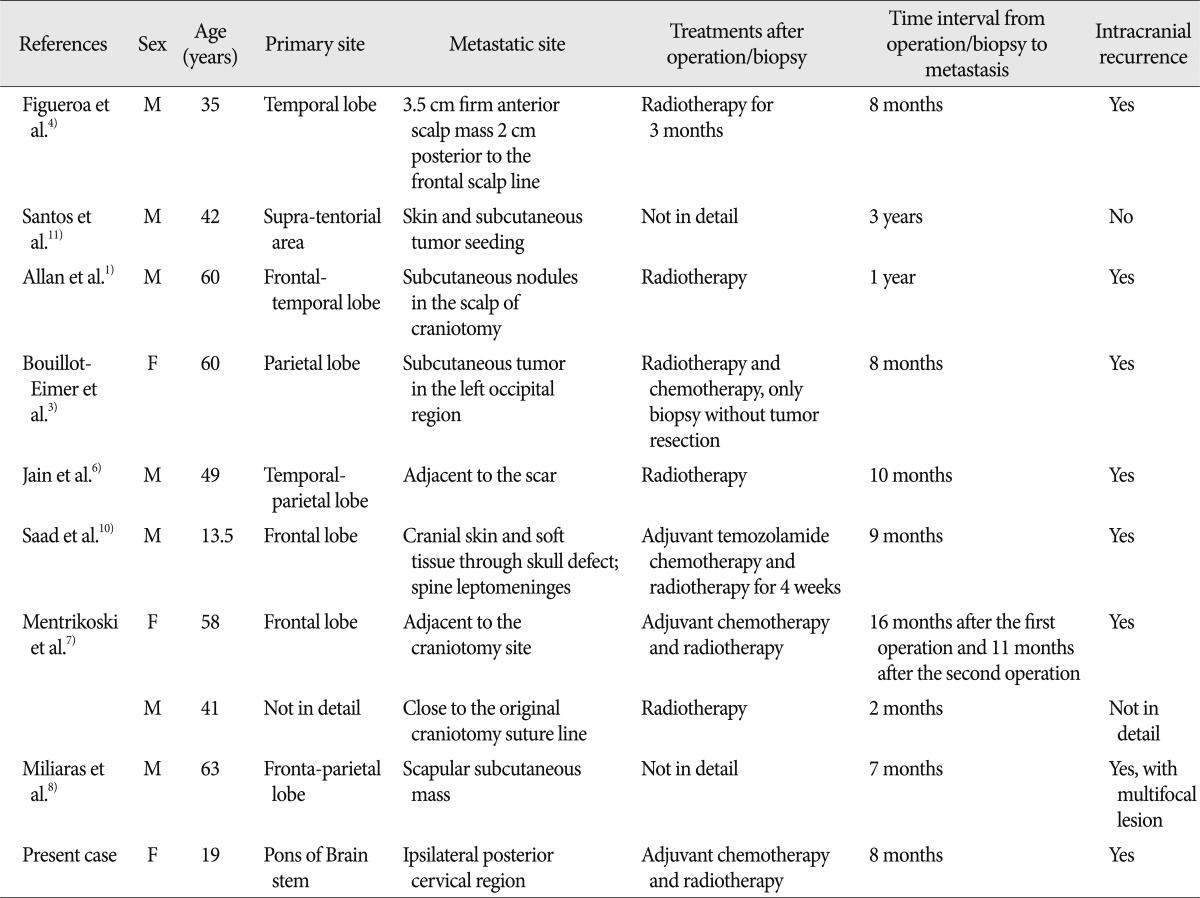
Glioblastoma multiforme (GBM) with subcutaneous metastases is extremely rare, and most proceeded after an invasive intracranial procedure (craniotomy or biopsy)1,3,4, 6-8,10,11). Here, we present 1 patient with pontine GBM metastasizing to subcutaneous tissue of the ipsilateral posterior cervical region. Furthermore, reasons for rarity, mechanisms, the diagnosis, and treatments of GBM with extracranial metastasis were reviewed.
A 19-year-old female patient presented with debility of left limbs for 2 days (d). Neurological examination revealed muscle strength of left limbs was graded IV. Cranial magnetic resonance (MR) showed a cystic mass measuring 2.0×2.5×3.0 cm located at the right part of pons; contrast MR (Fig. 1) showed the tumor was ring-enhanced with necrosis. The operation was performed via retro-sigmoid approach, and pathological examinations of the lesion confirmed as GBM (World Health Organization grade IV) that had variable histological features, such as mitosis, endothelial proliferation and necrosis (Fig. 2A-D). Immunohistochemical results showed that the tumor cells were positive for glial fibrillary acidic protein (GFAP) and S-100 protein (Fig. 2E, F). After operation, the patient was treated with three-dimensional conformal radiotherapy for 6 weeks (60 Gy in total with 30 fractions of 2 Gy) and concomitant temozolomide chemotherapy (75 mg/m2/daily for 42 d). After that, adjuvant temozolomide chemotherapy (150 mg/m2/daily for 5 d with every 28 d for 1 chemotherapy course) was suggested; however, the patient's family refused that because of financial reasons. 8 months after operation, the patient noticed an enlarging subcutaneous swelling at the left posterior cervical region On examination, a very firm, immobile subcutaneous swelling measured 2.5×3.0 cm was palpated. Follow-up MR (Fig. 3) scan showed that recurrence of pontine GBM, and an enhanced subcutaneous swelling at the left posterior cervical region was evident on magnetic resonance imaging. The swelling was resected, and pathological examinations, immunohistochemical results showed the same characteristics as the primary intracranial GBM cells (Fig. 4). Thus, a diagnosis of GBM metastasizing to the cervical subcutaneous tissue was rendered. As a result, temozolomide chemotherapy (150 mg/m2/daily for 5 d) and radiotherapy was administrated to the patient. 6-month-long follow up showed that there was no recurrence of the cervical subcutaneous tumor.
We feel that reporting of this rare case is of great significance for two reasons:
1) GBM with subcutaneous metastasis is extremely rare, and it is often misdiagnosed as primary subcutaneous tumor, which could delay effective treatments; and
2) Knowledge of a prior intracranial GBM, pathological examinations and immunocytochemical tests with markers typically expressed by GBM are critical for accurate diagnosis.
Although being exceedingly aggressive, GBM extremely rarely metastasize to the subcutaneous tissue. Including of our case described previously, only 10 cases with GBM subcutaneous metastases have been reported (Table 1)1,3,4,6-8,10,11).
Some scholars ascribed the rarity of GBM extracranial metastasis to the short survival of the disease2). Other scholars suggested that GBM are prevented from metastasizing because of lack of true lymphatics in the brain, the relatively impassable dura, extracelluar matrix, and tough basement membrane surrounding intracerebral blood vessels7). However, craniotomy breaches the brain's innate defensive systems, enabling tumor cells to gain entry to the lymphatic system, meningeal and vertebral venous system at the dural or intracerebral level2). It was reported that most of the GBM metastases occurred after craniotomy or stereotactic biopsies or intraventricular shunts2).
In the present case, we supposed the subcutaneous swelling could be spread through local dissemination since the swelling was just located 3 cm below the original suture line. However, homogenous metastasis, possibly through venous system could not be eliminated, as the swelling acquired a close relationship with the external jugular vein. In addition, it could not be excluded that the subcutaneous metastasis was spread through cerebrospinal fluid (CSF) dissemination, as the retrosigmoid approach is notoriously prone to leaking, and the CSF easily leak into the subcutaneous space, through the retrosigmoid opening into the sternocleidomastoid, and rectus capitus lateralis muscles. Overall, it may be difficult or impossible to distinguish that the true metastatic swelling was spread by local dissemination or CSF dissemination or hematogenous metastasis after operation in the case of lesions of subcutaneous tissue in close proximity to the craniotomy site.
As GBM with subcutaneous metastasis is rare, a clinical history of a pre-existing glioma is critical for accurate diagnosis. Knowledge of a prior intracranial GBM lead us to evaluate the tumor with immunocytochemical markers typically expressed by GBM, such as GFAP, S-100, helping to confirm the diagnosis7). GFAP-positivity indicated it could originate from intracranial astrocyte, which is of great importance for the diagnosis of metastatic intracranial glioma. Therefore, pathological and immunocytochemical examinations of the tumor after surgical resection or biopsy are of vital importance for the diagnosis of GBM metastasis. However, in the case of extracranial subcutaneous metastasis, a differential diagnosis with other small cell carcinoma, poorly differentiated carcinoma and neuroblastoma should also be considered5).
The standard therapy for GBM consists of surgical resection to the extent that is feasible safely, followed by radiotherapy plus concomitant and adjuvant temozolomide chemotherapy13). For the soft-tissue metastases, surgical resection or biopsy is recommended to confirm the extracranial metastasis, followed by chemotherapy and radiotherapy14). Steinbok et al.12) reported that cervical tumor decreased in size, indicating sensitivity to lomustine, but intracranial tumor was not responded. Possible explanations of this phenomenon are inadequate drug delivery to the intracranial tumor, different sensitivity of intracranial and extracranial tumors to the drug, or a combination of these factors9). In the present case, after resecting the subcutaneous swelling, temozolomide chemotherapy and radiotherapy was administrated to the patient immediately, and 6-month-long follow-up showed that the outcome was good without recurrence of the subcutaneous tumor. We suggest that surgical resection of GBM subcutaneous metastasis to the extent, followed by chemotherapy and radiotherapy, could be the best strategy of treatments for the patients.
In summary, GBM with subcutaneous extracranial metastasis is extremely rare. Knowledge of a prior intracranial GBM, pathological and immunocytochemical examinations with markers typically expressed by GBM are vital important for the diagnosis of GBM metastasis. Surgical resection of subcutaneous swelling, followed by chemotherapy and radiotherapy, could be the best strategy of treatment for the patients with GBM subcutaneous metastasis.
References
1. Allan RS. Scalp metastasis from glioblastoma. J Neurol Neurosurg Psychiatry. 2004; 75:559. PMID: 15026496.
2. Ates LE, Bayindir C, Bilgic B, Karasu A. Glioblastoma with lymph node metastases. Neuropathology. 2003; 23:146–149. PMID: 12777104.

3. Bouillot-Eimer S, Loiseau H, Vital A. Subcutaneous tumoral seeding from a glioblastoma following stereotactic biopsy : case report and review of the literature. Clin Neuropathol. 2005; 24:247–251. PMID: 16320817.
4. Figueroa P, Lupton JR, Remington T, Olding M, Jones RV, Sekhar LN, et al. Cutaneous metastasis from an intracranial glioblastoma multiforme. J Am Acad Dermatol. 2002; 46:297–300. PMID: 11807444.

5. González Cámpora R, Otal Salaverri C, Vázquez Ramirez F, Salguero Villadiego M, Galera Davidson H. Metastatic glioblastoma multiforme in cervical lymph nodes. Report of a case with diagnosis by fine needle aspiration. Acta Cytol. 1993; 37:938–942. PMID: 8249517.
6. Jain N, Mirakhur M, Flynn P, Choudhari KA. Cutaneous metastasis from glioblastoma. Br J Neurosurg. 2005; 19:65–68. PMID: 16147588.

7. Mentrikoski M, Johnson MD, Korones DN, Scott GA. Glioblastoma multiforme in skin : a report of 2 cases and review of the literature. Am J Dermatopathol. 2008; 30:381–384. PMID: 18645311.

8. Miliaras G, Tsitsopoulos PP, Markoula S, Kyritsis A, Polyzoidis KS, Malamou-Mitsi V. Multifocal glioblastoma with remote cutaneous metastasis : a case report and review of the literature. Cent Eur Neurosurg. 2009; 70:39–42. PMID: 19191206.

9. Moon KS, Jung S, Lee MC, Kim IY, Kim HW, Lee JK, et al. Metastatic glioblastoma in cervical lymph node after repeated craniotomies : report of a case with diagnosis by fine needle aspiration. J Korean Med Sci. 2004; 19:911–914. PMID: 15608410.

10. Saad AG, Sachs J, Turner CD, Proctor M, Marcus KJ, Wang L, et al. Extracranial metastases of glioblastoma in a child : case report and review of the literature. J Pediatr Hematol Oncol. 2007; 29:190–194. PMID: 17356401.
11. Santos AV, Saraiva PF, Santiago B. [Extracranial metastasis of glioblastoma multiforme]. Acta Med Port. 2003; 16:209–211. PMID: 12868404.
12. Steinbok P, Dolman CL, Goldie JH. Variation in response to CCNU of glioblastoma multiforme in brain and cervical lymph node. Case report. J Neurosurg. 1985; 62:918–921. PMID: 2987441.

13. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.

14. Zhen L, Yufeng C, Zhenyu S, Lei X. Multiple extracranial metastases from secondary glioblastoma multiforme : a case report and review of the literature. J Neurooncol. 2010; 97:451–457. PMID: 19898745.

Fig. 1
Cranial magnetic resonance pre-operation. A-C shows a cystic mass measuring 2.0×2.5×3.0 cm located at the right part of pons and the tumor is ring-enhanced with necrosis; the fourth ventricle is compressed obviously as to suspect glioblastoma multiforme.
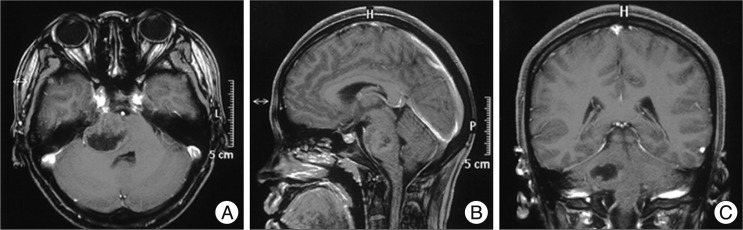
Fig. 2
Pathological and Immunohistochemical examinations of the primary tumor. A (HE×50) shows a low-power view of the tumor with endothelial proliferation and necrosis; B-C (HE×200), D (HE×400) shows variable histological features, such as endothelial proliferation, necrosis and mitosis; Immunohistochemical results (E-F×200) shows that the tumor cells are positive for glial fibrillary acidic protein (E×200) and S-100 protein (F×200).
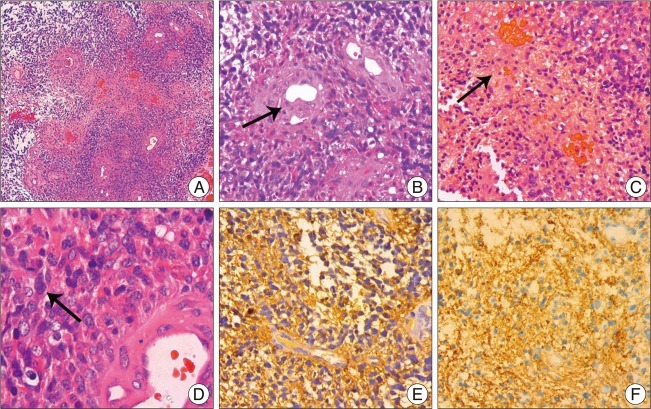
Fig. 3
Flollow-up magnetic resonance 8 months after operation. A-B shows that recurrence of pontine glioblastoma multiforme extending to the ipsilateral cerebellar hemisphere; C-F shows that an enlarging subcutaneous swelling at the left posterior cervical region was evident. The swelling is enhanced obviously and had a close relationship with the external jugular vein, although without encasing or infiltrating it obviously.
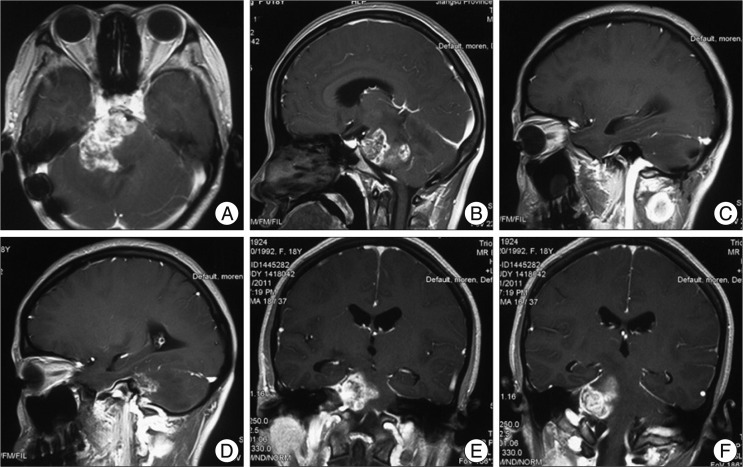
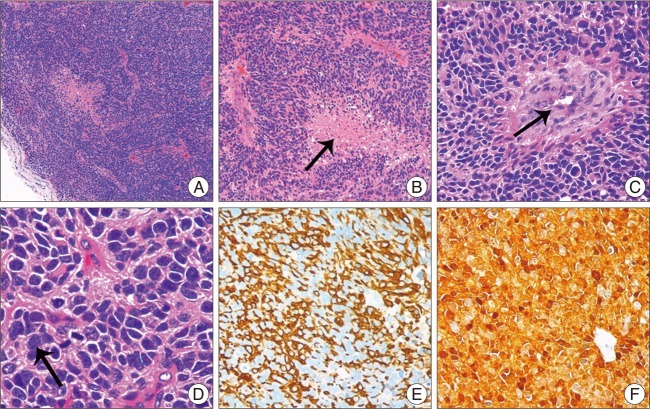
Fig. 4
Pathological and Immunohistochemical examinations of the subcutaneous swelling. A (HE×50) shows a low-power view features of the tumor cell; B-C (HE×200), D (HE×400) shows variable histological features, such as endothelial proliferation, necrosis and mitosis. When compared with the primary pathological examinations, mitosis and tumor necrosis were more frequent. Immunohistochemical results revealed that the tumor cells were strongly positive for glial fibrillary acidic protein (E×200) and S-100 protein (F×200), which showed the same characteristics as the primary intracranial glioblastoma multiforme cells.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download