Abstract
Objective
The objective of study is to evaluate the incidence of leptomeningeal carcinomatosis (LMC) in breast cancer patients with parenchymal brain metastases (PBM) and clinical risk factors for the development of LMC.
Methods
We retrospectively analyzed 27 patients who had undergone surgical resection (SR) and 156 patients with whole brain radiation therapy (WBRT) as an initial treatment for their PBM from breast cancer in our institution and compared the difference of incidence of LMC according to clinical factors. The diagnosis of LMC was made by cerebrospinal fluid cytology and/or magnetic resonance imaging.
Results
A total of 27 patients (14%) in the study population developed LMC at a median of 6.0 months (range, 1.0-50). Ten of 27 patients (37%) developed LMC after SR, whereas 17 of 156 (11%) patients who received WBRT were diagnosed with LMC after the index procedure. The incidence of LMC was significantly higher in the SR group compared with the WBRT group and the hazard ratio was 2.95 (95% confidence interval; 1.33-6.54, p<0.01). Three additional factors were identified in the multivariable analysis : the younger age group (<40 years old), the progressing systemic disease showed significantly increased incidence of LMC, whereas the adjuvant chemotherapy reduce the incidence.
The incidence of parenchymal brain metastasis (PBM) from primary systemic cancer has been increasing along with prolonged survival of the patients with cancer. Recent report showed that up to 30% of cancer patients can be expected to develop PBM according to the types of primary cancer6,12). A population-based estimate of the incidence proportion of PBM was highest for lung cancer followed by melanoma, renal-, and breast cancer3).
However, leptomeningeal carcinomatosis (LMC), which is one of terminal manifestations of central nervous system (CNS) metastasis from systemic malignancy, remains incurable without a discernible advance in treatment over the past decades. LMC also has been diagnosed more frequently with the advancement of neuroimaging and prolonged survival of cancer patients8,13,29). Among the pathogenesis of LMC, spread of cancer cells followed by surgical resection (SR) has been suggested from several clinical observations of solid tumors including malignant brain tumors2,11,16,22,23,34). Although the overall incidence of LMC is difficult to estimate according to PBM status, it is apparent that the primary cancer showing a high incidence of PBM, such as lung cancer, breast cancer or melanoma, develops into a high proportion of LMC among those cancer patients5,35).
Several reports suggested that the risk for development of LMC may increase in patients with PBM who underwent SR compared with non-surgical treatment, and at the same time, the risk may also be affected by factors such as location of the tumor, treatment modality and the histology of primary cancer21,23,24,30-33). However, to our knowledge, neither a controlled-prospective study nor any study which attempted to adjust these variables to evaluate the significant risk factors for LMC has been reported.
Breast cancer is known to be the second most common cancer associated with PBM and is also, one of the most common primary cancers that causes LMC5,6,38). Thus, we retrospectively collected data on patients with PBM from one primary histology of breast cancer. The primary objective of the study was to estimate the incidence of LMC after SR or whole brain radiation therapy (WBRT) as the initial treatment in a cohort of breast cancer patients with PBM. Further, we examined if SR confers a higher risk for the development of LMC than WBRT in these patients, and also, analyzed the influence of the above mentioned clinical factors on the development of LMC.
From our institutional medical record database, we identified 274 cases of SR or WBRT as the initial treatment for PBM from breast cancer between August, 2001 and December, 2010. Eleven breast cancer patients, who received radiosurgery as the initial treatment for their PBM, were not included in this cohort.
Patients were excluded if they were diagnosed with LMC before the index procedure (12 patients), did not have a MRI follow-up at our institution after the index procedure (76 patients), or had incomplete data (3 patients) on entities under study. A total 183 patients met the eligibility criteria and are included in this analysis. Twenty-seven patients (15%) underwent SR of their tumors, and the remaining 156 patients (85%) received WBRT. Among 27 SR group, 11 patients underwent WBRT within one month after SR as an adjuvant treatment, 10 patients received WBRT after local or distant recurrence of their brain metastasis, and the remaining 6 patients were observed without WBRT. Patients were followed up with MRI every 3 to 6 months, until the patients died or no longer returned to our hospital.
We reviewed the following factors from medical records and conventionally categorized them for the analysis : type of index procedure (SR or WBRT), age, time from diagnosis to brain metastasis, volume of tumor, number of brain metastatic lesions (single or multiple), location (supratentorial or infratentorial) of the brain metastatic lesion, adjuvant chemotherapy, Karnofsky Performance Status (KPS) scale and systemic disease status (no evidence of disease, stable, or progressing).
The primary outcome was the incidence of LMC, as diagnosed by cytological CSF analysis or neuroimaging findings such as "clear leptomeningeal enhancement extending into the cerebral sulci or into the folia of the cerebellum, spinal cord, cauda equina or subependyma"13). All patients were evaluated by brain MRI at least once for monitoring local or cerebral recurrence. Spinal MRI and CSF cytology was examined only if the patient had symptoms of LMC. Time to event (LMC) was defined as the time from the index procedure to the diagnosis of LMC or the last follow-up image. WBRT group patients were censored when they received SR for the management of recurrent tumor to avoid overlapped exposure to higher risk.
The differences in the distributions of categorical variables for SR and WBRT groups as well as the incidence of LMC were analyzed using the chi-square test or Fisher exact test, as appropriate. Continuous variables were tested using Student's t-test or Wilcoxon test. A p-value of 0.05 or less was considered statistically significant. For the analysis of the cumulative incidences of LMC, patients were censored at the end of observation or lost to follow-up. In the univariable analysis, the Cox proportional hazard model was used to estimate the hazard and corresponding 95% confidence interval (CI) of developing LMC for each of the risk factors. Factors that showed at least marginal significance from the univariable analysis (p-value <0.2) were included in the multivariable analysis to account interrelationships among the variables.
Characteristics of patients who underwent SR or WBRT for their PBM are presented in Table 1. The median follow-up (of image) after index procedure was 6.0 months (range, 1-51 months), and the follow-up periods were not different according to the treatment modality with a median of 5.8 months for the WBRT group and 8.0 months for the SR group. The median survival of SR group was 14.5 months (95% CI; 10.5-28.7) and that of WBRT only was 9.4 months (95% CI; 7.4-11.9). However, this apparent difference of median survival between SR group and WBRT group was not statistically significant (p>0.05).
The median age of the patients at the time of the index procedure was 52 years (range, 28-80 years), and the median time from diagnosis of the primary cancer to brain metastasis was 41 months (range, 1-271 months). The median tumor volume was 4.0 cm3 (range, 0.01-105.0 cm3), and it was significantly different between two modes of treatment : the median tumor volume of the SR group was 10.9 cm3 while that of the WBRT group was 3.1 cm3 (p<0.001). Fifty-eight patients (32%) had a single lesion at the time of index procedure while the remaining 125 patients (68%) had multiple metastases. Patients with a single lesion were more frequently treated by SR than those with multiple lesions (p<0.001). Supratentorial lesions constituted 28% of the patients, and an infratentorial lesion occurred in 72% of the patient. Infratentorial lesion includes the case with both supratentorial and infratentorial brain metastases. Adjuvant chemotherapy after the index procedure was performed in 103 patients (56%) KPS was equal or greater than 70 in 90% of the patients. At the time of the index procedure, 118 patients (64%) had 'progressive' systemic disease and 65 patients had 'stable' disease or 'no evidence of disease (NED)'.
LMC occurred in 27 out of 183 patients (14%) with the median time to LMC of 8.0 months (range, 2-26 months) (Fig. 1). LMC was diagnosed only by typical MRI findings for ten patients, only by cytology for two patients, and by both CSF cytology and MRI for the remaining 15 patients. After diagnosed with LMC, 15 patients received intra-CSF chemotherapy. Four patients received WBRT only with or without spinal radiation and the remaining 8 patients deferred any further treatment.
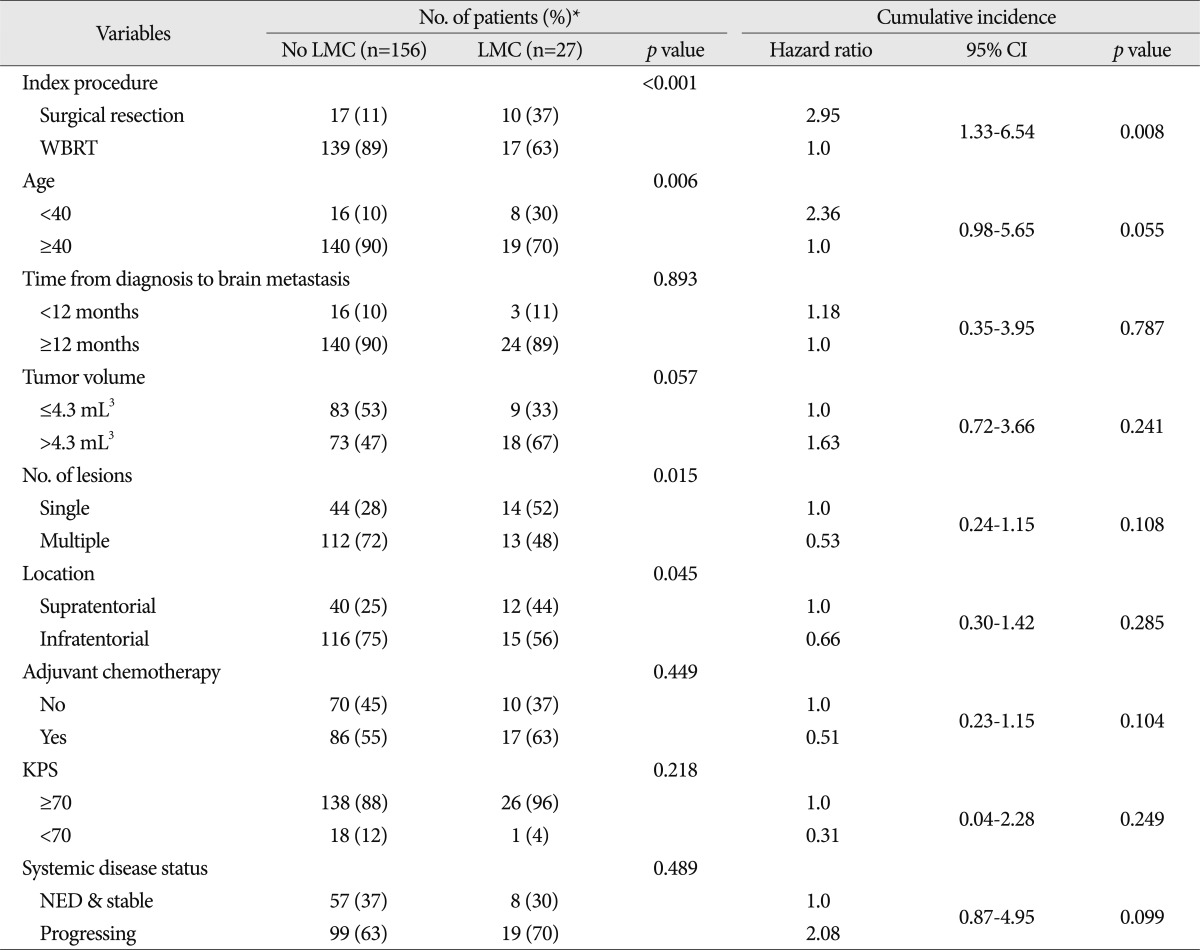
The distributions of the incidence of LMC according to various patients' characteristics and the hazard ratios of these factors based on cumulative LMC incidences are presented in Table 2.
The incidence of LMC was significantly higher in the SR group than the WBRT group, as 10 of 27 (37%) patients were diagnosed with LMC. The hazard ratio (HR) for development of LMC in the SR group compared to WBRT was 2.95 (95% CI, 1.33-6.54; p=0.008) (Fig. 2). The incidence of LMC was higher in the younger age group (<40 years old) compared with that in patients aged 40 years or older as 8 out of 24 younger age patients developed LMC after the index procedure. However, the difference was not statistically significant at 0.05 (HR=2.36, 95% CI, 0.98-5.65, p=0.055). The time from diagnosis to brain metastasis (<12 versus ≥12 months), tumor volume (≤4.0 versus >4.0 mL3), number of lesions (single versus multiple), location of tumor (supratentorial versus infratentorial), adjuvant chemotherapy, KPS (≥70 versus <70) and systemic disease status (NED or stable versus progressing) were not significantly associated with the incidence of LMC in univariable analysis.
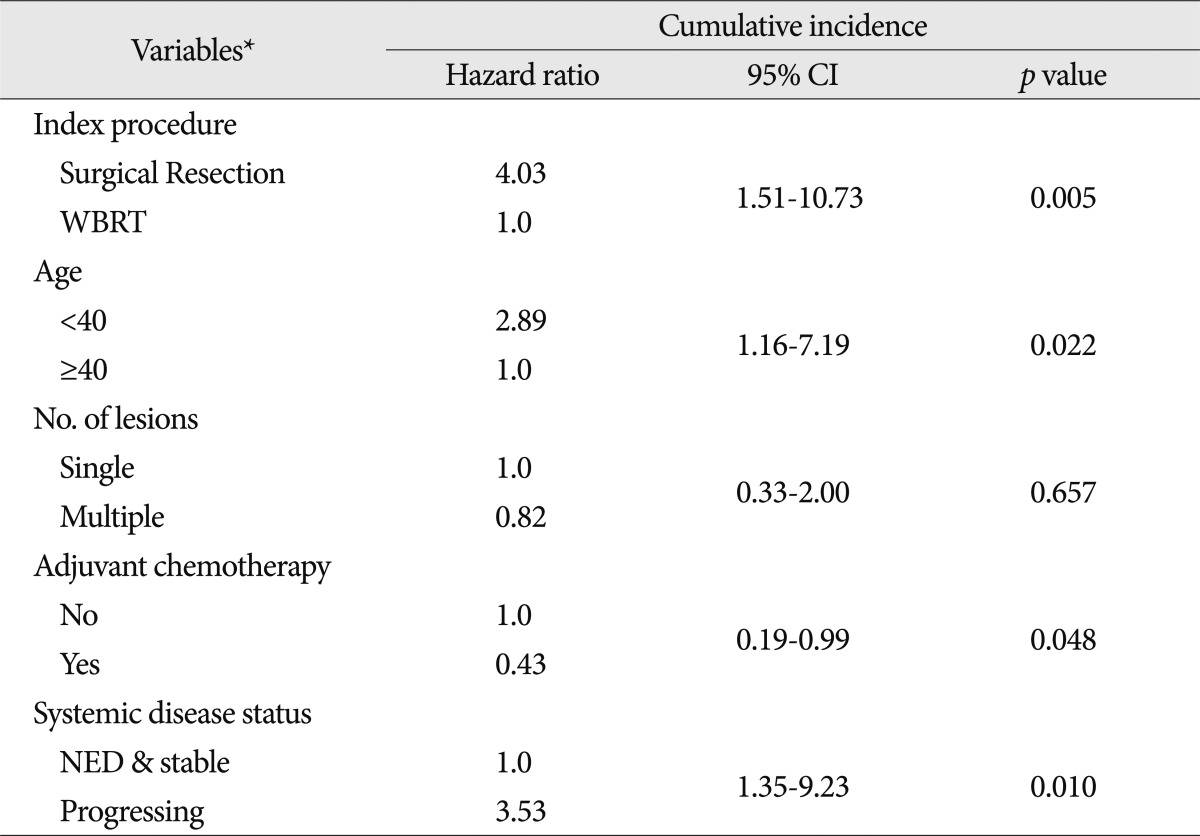
Among the factors analyzed univariately for the cumulative incidence of LMC, variables with a p-value less than 0.2 were included in the multivariable Cox proportional hazards model (Table 3).
Among the preoperative characteristics, the index procedure was still significant and the younger age group gained statistical significance in this multivariable analysis. The administration of adjuvant chemotherapy and NED/stable systemic disease status significantly reduced the cumulative incidence of LMC in multivariable analysis even though these variables were not significant at the p-value 0.05 in the univariable analysis. Compared to the WBRT group, the HR of the SR group was 4.03 (95% CI, 1.51-10.73; p=0.005). The HR of the younger age (<40) group was 2.89 (95% CI, 1.16-7.19; p=0.022), adjuvant chemotherapy group decreased the risk for development of LMC at a HR of 0.43 (95% CI, 0.19-0.99; p=0.048), whereas patients with progressing systemic disease showed increased risk of LMC at a HR of 3.53 compared with NED or stable systemic disease (95% CI, 1.35-9.23; p=0.010).
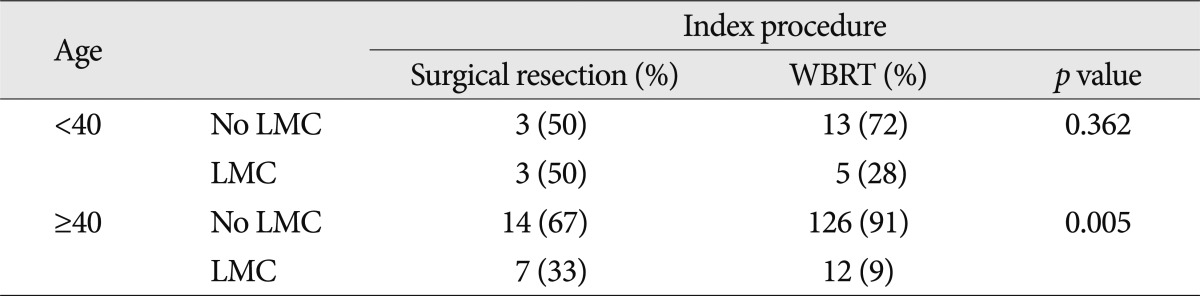
To evaluate the differential effect of treatment modality on the development of LMC according to the age group, we performed the subgroup analysis presented in Table 4. For the different modes of treatment, WBRT group showed lower incidence of LMC in the old age (≥40) group (Fisher's exact test, p=0.005), whereas the difference according to the index procedure became not significant in the young age (<40) group due to high incidence of LMC regardless of the index procedures.
LMC most often presents in patients with progressive systemic cancer. However, occasionally it occurs in the absence of systemic disease, and it can even be the first manifestation of cancer5). Thus, LMC can develop independently from systemic malignancy without PBM, but some studies reported that patients who underwent SR for their PBM had a higher incidence of LMC compared with that of patients received non surgical treatments31-33). It is unclear how PBM spreads and causes LMC, but we could observe the surgical spillage or the drop-metastasis of malignant brain tumors2,25,35). The incidence of LMC in patients with PBM compared with that in patients without PBM are yet to be studied, and even reports investigating the incidence of LMC in patients with PBM are rare17,18,30). Kim et al.17) reported that in 400 patients with CNS metastases of breast cancer, 318 patients (79.5%) had PBM only, 52 patients (13%) had both PBM and LMC, and 30 patients (7.5%) had LMC only. The number of breast cancer patients diagnosed with histologic confirmation during the same period was 10172 in that study, and based on this, we inferred the incidence of LMC in patients with PBM as 52/370 (14%), which was much higher than that in patients without PBM (30/9, 802, 0.3%). Lee et al.18) reported that the incidence of LMC among CNS metastases of breast cancer was 25% (68 of 272 patients), which was apparently higher than the known incidence proportion of LMC in breast cancer patients which is about to 1-5%5). However, this simple comparison should be understood with caution because many clinical factors including systemic disease status may affect the incidence.
The hematogenous pathway can be a route for both PBM and LMC as tumor cells overcome blood-brain and blood-CSF barrier28). Although it is not clear whether systemic chemotherapy can prevent either PBM or LMC, several studies indicated that systemic chemotherapy could prolong patients' survival with PBM from breast cancer and intra-CSF chemotherapy is currently considered the best treatment option for LMC14,17,26,28,36). It is noteworthy that systemic disease status and chemotherapy were significant factors affecting the incidence of LMC in our study.
In view of the poor prognosis of patients with LMC, early diagnosis of LMC with prompt initiation of treatment is important to prolong survival, especially in patients with good performance status and neurological function8). Physicians should be alerted to a differential risk of the condition depending on certain characteristics, and the method of treatment; early appropriate actions to prevent or treat LMC in higher-risk patients should be taken. Recent trends in diagnosing LMC with MRI are highly sensitive although it is not definitive13). A strong adherence of malignant cells to the leptomeninges or the presence of a focal rather than widespread leptomeningeal tumor, can contribute to false-negative cytological results15,37). Although the neuroimaging-based diagnosis of LMC without CSF cytology is not generally accepted as a definitive diagnosis, a consensus is reached to allow for the treatment of LMC diagnosed by radiographic evidence only in known cancer patients. In our study, 10 patients were diagnosed with LMC based only on an MRI. Four of them revealed a negative CSF finding and in the remaining 6 patients, CSF testing was not performed.
It is known that certain biological features of breast cancer affect clinical finding including LMC. Several reports have suggested a higher risk for brain metastasis in HER-2 positive and triple negative (TN) breast subtypes than in hormone receptor (HR) positive subtypes6,19,20). Lee et al.18) reported a different distribution of subtypes of breast cancer between PBM with LMC and PBM without LMC, and a worse prognosis of patients with TN. However, the direct comparison of incidence of LMC according to the subtypes of breast cancer was not made.
Several studies suggested that mechanical spread of tumor cells to the CSF space could contribute to the development of LMC. Some studies reported an increased incidence of LMC after posterior fossa tumor surgery compared with supratentorial lesions25,35). Other studies observed a higher incidence of LMC after SR compared with non-surgical treatment31-33). Norris et al.25) suggested that the more chance of CSF exposure in patients receiving posterior fossa tumor removal was responsible for the increased development of LMC. Suki et al.33) reported increased incidence of LMC in patients with PBM, whose tumor was removed in a piecemeal manner than en-bloc excision. Thus, authors investigated the incidence of LMC after SR of PBM according to the proximity to CSF pathway and suggested the PBM not entirely surrounded by brain parenchyma should be removed in caution not to spill the tumor by piecemeal removal or using Cavitron ulatrsonographic aspirator1). In our study, the SR group showed a significantly higher incidence of LMC than the WBRT group, which is in accordance with previous finding. However, the PBM location whether or not the lesions involved posterior fossa, did not affect the risk of development of LMC. This result may reflect not tumor location itself but a chance for CSF exposure to be responsible for development of LMC.
Known prognostic factors for the survival of breast cancer patients include axillary nodal status, tumor size/type/grade, lymphatic/vascular invasion, proliferation markers, ethnicity and patient age at diagnosis. Many studies, which evaluated the influence of age on outcome in breast cancer have been small and had conflicting results. Two relatively large trials have, however, demonstrated a worse prognosis for patients younger than 35 years of age, even after adjustment for other prognostic factors4,39). Studies suggested different and aggressive biological behaviors of younger age breast cancer. A different distribution of molecular phenotypes in the population of young women with breast cancer compared to the general population of women with breast cancer was reported7). Young age appears to be an independent risk factor and the median age of patients with brain metastases is about 5 years younger than those without brain metastases6). In our study, an age of younger than 40 years was an independent risk factor for development of LMC in patients with PBM from breast cancer. This propensity is in accordance with the observation of Lee et al.18), where the age of PBM from breast cancer patients with LMC was significantly younger than those without LMC although the variables were not adjusted.
The role of systemic chemotherapy to prevent CNS metastasis has not yet been proven. However, a recent study reported a favorable outcome showing significantly prolonged survival in patients with PBM from breast cancer who received chemotherapy, compared with the patients without chemotherapy16). These finding leave a possibility that the chemotherapy directly reduce PBM from breast cancer or LMC, in addition to prolonging patient's survival by decreasing the systemic cancer burden. In our study, the patients in a direction of reduced systemic cancer burden (i.e. patients with stable or NED of systemic cancer and patients with systemic chemotherapy) showed a decreased risk for the development of LMC after treatment for their PBM from breast cancer.
The role of WBRT for preventing CNS recurrence after SR of PBM was proven in a randomized controlled clinical trial27). However, it is unclear whether or not this "CNS recurrence" included LMC, and we could not find any study that compared not the local recurrence rate, but the risk of LMC after SR in patients with or without WBRT9,10). An 11% incidence of LMC in the WBRT only group in our study is the first presented figure indicating the natural risk for the development of LMC without SR. But in this cohort, we did not have a group of patients, who had PBM from breast cancer without WBRT. Thus, to evaluate the role of WBRT for preventing the development of LMC, a further well controlled cohort study is needed.
This retrospective analysis revealed an increased risk for the development of LMC after SR of PBM from breast cancer compared with WBRT. The young age (<40) and systemic cancer burden in terms of progressing systemic could be additional risk factors for LMC, whereas continued systemic chemotherapy after the index procedure may reduce the incidence of LMC in patients with PBM from breast cancer.
References
1. Ahn JH, Lee SH, Kim S, Joo J, Yoo H, Lee SH, et al. Risk for leptomeningeal seeding after resection for brain metastases : implication of tumor location with mode of resection. J Neurosurg. 2012; 116:984–993. PMID: 22339161.

2. Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK, Jeun SS. The clinical features of spinal leptomeningeal dissemination from malignant gliomas. J Korean Neurosurg Soc. 2011; 49:334–338. PMID: 21887390.

3. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004; 22:2865–2872. PMID: 15254054.

4. Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol. 2011; 21:26–34. PMID: 21134651.

6. Cheng X, Hung MC. Breast cancer brain metastases. Cancer Metastasis Rev. 2007; 26:635–643. PMID: 17717635.

7. Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012; 131:1061–1066. PMID: 22080245.

8. DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. J Neurooncol. 1998; 38:245–252. PMID: 9696379.
9. DeAngelis LM, Mandell LR, Thaler HT, Kimmel DW, Galicich JH, Fuks Z, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989; 24:798–805. PMID: 2473409.

10. Dosoretz DE, Blitzer PH, Russell AH, Wang CC. Management of solitary metastasis to the brain : the role of elective brain irradiation following complete surgical resection. Int J Radiat Oncol Biol Phys. 1980; 6:1727–1730. PMID: 7239992.

11. Fortner JG. Inadvertent spread of cancer at surgery. J Surg Oncol. 1993; 53:191–196. PMID: 8331942.

12. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011; 22:1–6. vPMID: 21109143.

13. Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995; 38:51–57. PMID: 7611725.

14. Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010; 21:2183–2187. PMID: 20430906.

15. Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF) : the meaning of a positive CSF cytology. Neurology. 1979; 29:1369–1375. PMID: 573381.

16. Grabb PA, Albright AL, Pang D. Dissemination of supratentorial malignant gliomas via the cerebrospinal fluid in children. Neurosurgery. 1992; 30:64–71. PMID: 1738457.

17. Kim HJ, Im SA, Keam B, Kim YJ, Han SW, Kim TM, et al. Clinical outcome of central nervous system metastases from breast cancer : differences in survival depending on systemic treatment. J Neurooncol. 2012; 106:303–313. PMID: 21938531.

18. Lee S, Ahn HK, Park YH, Nam do H, Lee JI, Park W, et al. Leptomeningeal metastases from breast cancer : intrinsic subtypes may affect unique clinical manifestations. Breast Cancer Res Treat. 2011; 129:809–817. PMID: 21785952.

19. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer : high incidence of central nervous system metastases. Cancer. 2008; 113:2638–2645. PMID: 18833576.

20. Lin NU, Winer EP. Brain metastases : the HER2 paradigm. Clin Cancer Res. 2007; 13:1648–1655. PMID: 17363517.
21. Mahajan A, Borden J, Tsai JS. Carcinomatous meningitis : are surgeryand gamma knife radiosurgery treatment risk factors? J Neurosurg. 2002; 97:441–444. PMID: 12507072.

22. Mayo WJ. Grafting and traumatic dissemination of carcinoma in the course of operations for malignant disease. JAMA. 1913; 60:512–513.

23. Mirimanoff RO, Choi NC. Intradural spinal metastases in patients with posterior fossa brain metastases from various primary cancers. Oncology. 1987; 44:232–236. PMID: 3039433.

24. Mirimanoff RO, Choi NC. The risk of intradural spinal metastases in patients with brain metastases from bronchogenic carcinomas. Int J Radiat Oncol Biol Phys. 1986; 12:2131–2136. PMID: 3793550.

25. Norris LK, Grossman SA, Olivi A. Neoplastic meningitis following surgical resection of isolated cerebellar metastasis : a potentially preventable complication. J Neurooncol. 1997; 32:215–223. PMID: 9049883.
26. Pace A, Fabi A. Chemotherapy in neoplastic meningitis. Crit Rev Oncol Hematol. 2006; 60:194–200. PMID: 16949298.

27. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500. PMID: 2405271.

28. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004; 15(Suppl 4):iv285–iv291. PMID: 15477323.

29. Rosen ST, Aisner J, Makuch RW, Matthews MJ, Ihde DC, Whitacre M, et al. Carcinomatous leptomeningitis in small cell lung cancer : a clinicopathologic review of the National Cancer Institute experience. Medicine (Baltimore). 1982; 61:45–53. PMID: 6276648.
30. Seute T, Leffers P, ten Velde GP, Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer. 2005; 104:1700–1705. PMID: 16080173.

31. Siomin VE, Vogelbaum MA, Kanner AA, Lee SY, Suh JH, Barnett GH. Posterior fossa metastases : risk of leptomeningeal disease when treated with stereotactic radiosurgery compared to surgery. J Neurooncol. 2004; 67:115–121. PMID: 15072456.

32. Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008; 108:248–257. PMID: 18240919.

33. Suki D, Hatiboglu MA, Patel AJ, Weinberg JS, Groves MD, Mahajan A, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009; 64:664–674. discussion 674-676. PMID: 19197219.

34. Umpleby HC, Williamson RC. Anastomotic recurrence in large bowel cancer. Br J Surg. 1987; 74:873–878. PMID: 3311277.

35. van der Ree TC, Dippel DW, Avezaat CJ, Sillevis Smitt PA, Vecht CJ, van den Bent MJ. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999; 66:225–227. PMID: 10071105.

36. Waki F, Ando M, Takashima A, Yonemori K, Nokihara H, Miyake M, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009; 93:205–212. PMID: 19043775.

37. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors : experience with 90 patients. Cancer. 1982; 49:759–772. PMID: 6895713.

38. Yap HY, Yap BS, Tashima CK, DiStefano A, Blumenschein GR. Meningeal carcinomatosis in breast cancer. Cancer. 1978; 42:283–286. PMID: 667799.

39. Yildirim E, Dalgiç T, Berberoğlu U. Prognostic significance of young age in breast cancer. J Surg Oncol. 2000; 74:267–272. PMID: 10962458.

Fig. 1
Kaplan-Meier curve for time to development of LMC after treatment of the parenchymal brain metastases plotted for 183 patients in our study. Y-axis represents the proportion of patients without LMC at each follow-up time at the X-axis. 27 patients developed LMC (14%) during the follow-up. LMC : leptomeningeal carcinomatosis.
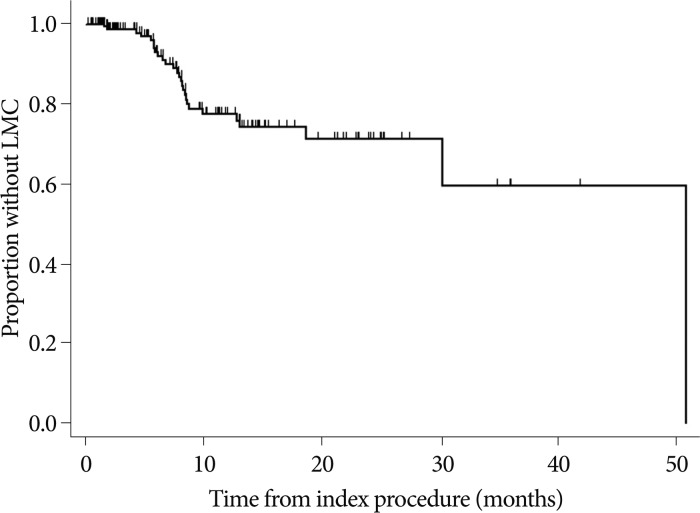
Fig. 2
Kaplan-Meier curves for time to development of LMC after treatment of the parenchymal brain metastases according to index procedure. Y-axis represents the proportion of patients without LMC at each follow-up time at the X-axis. Patients in the surgical resection (n=27) conferred greater risk for developing LMC compared to those in the WBRT (n=156) (HR estimated by the Cox proportional hazard model=2.95, 95% confidence interval, 1.33-6.54, p=0.008). LMC : leptomeningeal carcinomatosis, WBRT : whole brain radiation therapy, HR : hazard ratio.
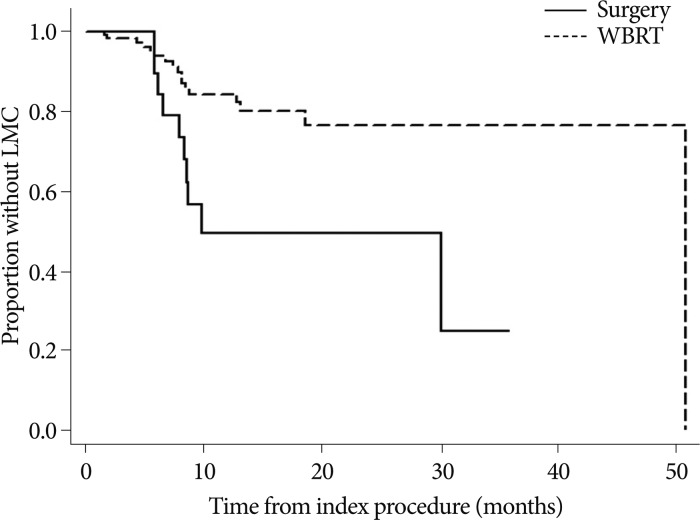
Table 1
Characteristics of patients with who underwent surgical resection or whole brain radiation therapy for brain metastases of breast cancer
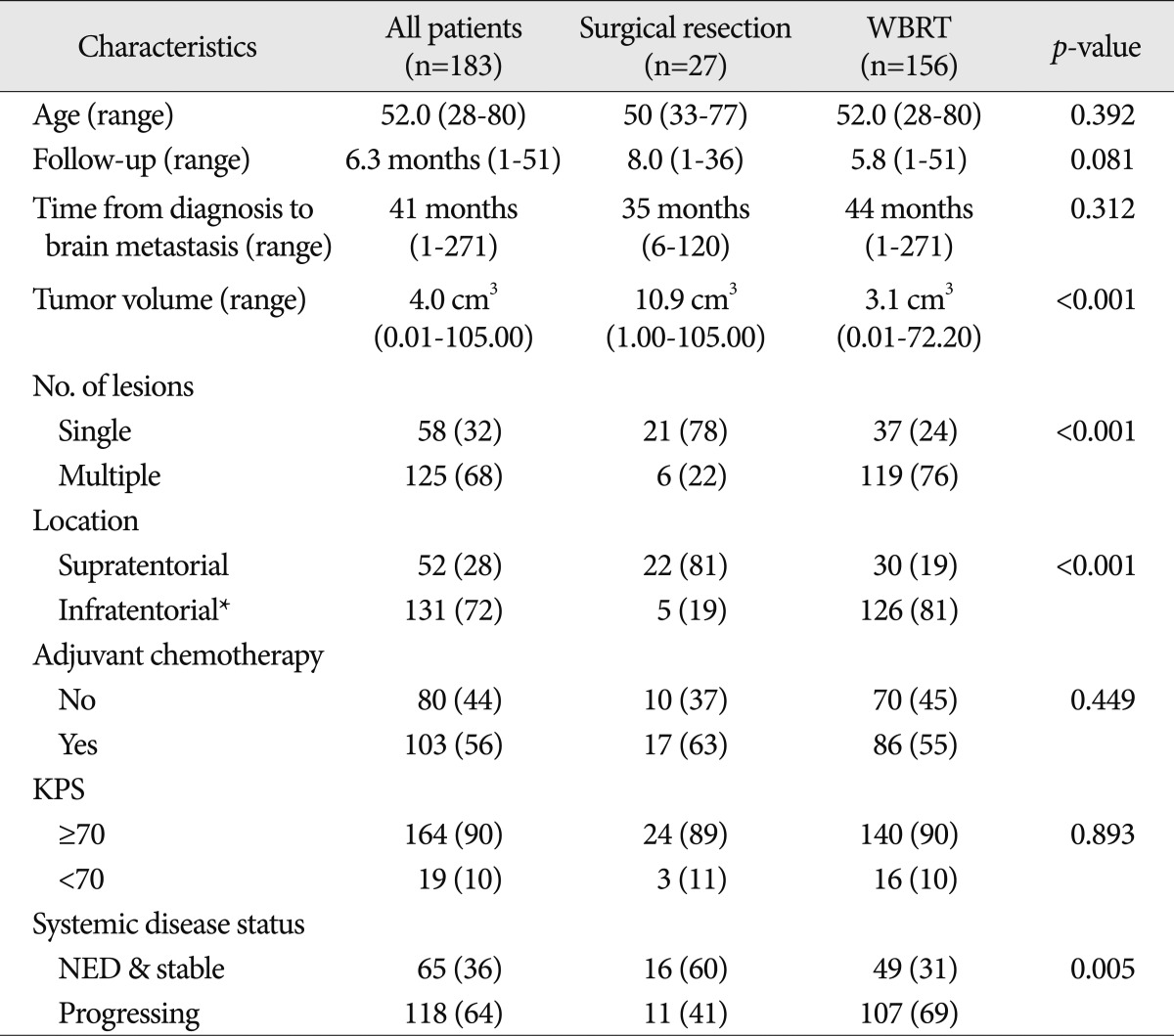
Table 2
Univariable analysis of factors possibly affecting the cumulative incidence of leptomeningeal seeding after surgical resection or whole brain radiotherapy for brain metastases




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download