Abstract
Objective
This study was conducted to assess the clinical significance of traumatic brain stem injury (TBSI) reflected on Glasgow Coma Score (GCS) and Glasgow Outcome Score (GOS) by various clinical variables.
Methods
A total of 136 TBSI patients were selected out of 2695 head-injured patients. All initial computerized tomography and/or magnetic resonance imaging studies were retrospectively analyzed according to demographic- and injury variables which result in GCS and GOS.
Results
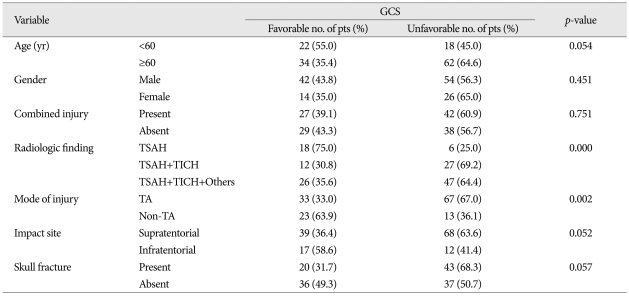
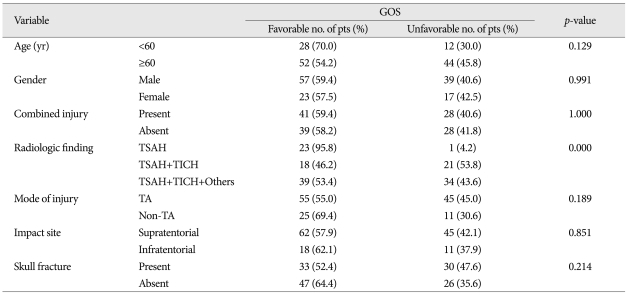
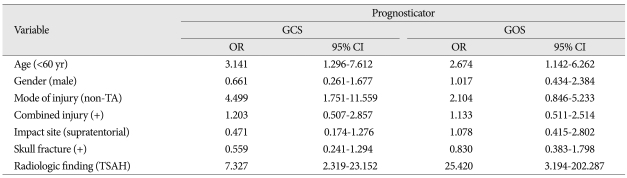
In univariate analysis, mode of injury showed a significant effect on combined injury (p<0.001), as were the cases with skull fracture on radiologic finding (p<0.000). The GCS showed a various correlation with radiologic finding (p<0.000), mode of injury (p<0.002), but less favorably with impact site (p<0.052), age (p<0.054) and skull fracture (p<0.057), in order of statistical significances. However, only GOS showed a definite correlation to radiologic finding (p<0.000). In multivariate analysis, the individual variables to enhance an unfavorable effect on GCS were radiologic finding [odds ratio (OR) 7.327, 95% confidence interval (CI)], mode of injury (OR; 4.499, 95% CI) and age (OR; 3.141, 95% CI). Those which influence an unfavorable effect on GOS were radiologic finding (OR; 25.420, 95% CI) and age (OR; 2.674, 95% CI).
Conclusion
In evaluation of TBSI on outcome, the variables such as radiological finding, mode of injury, and age were revealed as three important ones to have an unfavorable effect on early stage outcome expressed as GCS. However, mode of injury was shown not to have an unfavorable effect on late stage outcome as GOS. Among all unfavorable variables, radiological finding was confirmed as the only powerful prognostic variable both on GCS and GOS.
The incidence of traumatic brain stem injury (TBSI) varied 8.8% to 52%9,13) and TBSI might induce a serious impact on brain tissue as a form of diffuse axonal injury (DAI). Poor prognosis was a common feature following severe traumatic brain injury, and furthermore, it was more common in those with TBSI. However, TBSI is no more considered as powerful indicator to predict bad outcome. Many clinical case reports were publicized to elucidate the causal relationship between TBSI and outcome by means of radiologic findings and anatomical studies, and now some aspects of its pathomechanism could be revealed. Therefore, we conducted this study to reappraise the correlationship among clinical variables, such as impact site on scalp and radiologic finding on Glasgow Coma Score (GCS) and Glasgow Outcome Score (GOS).
The data were retrospectively collected from the clinical records of 136 TBSI cases among consecutive 2695 head-injured patients who had admitted from January 2004 to December 2008. In case of outpatient and the rest of patients, follow-up records were reviewed and telephone interviews were used. The exclusion criteria for data selection in this study were episode of shock state, history of alcohol intoxication, episode of blood dyscrasia, and previous history of head injury.
The correlation was analyzed among seven clinical variables (age, gender, mode of injury, combined injury, impact site on scalp, radiologic finding inclusive of skull fracture), which were reflected on GCS as an early prognosticator and GOS as a late one. Initially, the correlation was analyzed among seven clinical variables using univariate and multivariate analyses. The chi square test was used for the univariate analysis and logistic regression model was used for the multivariate analysis. p-values less than 0.05 were considered as statistically significant for all measures.
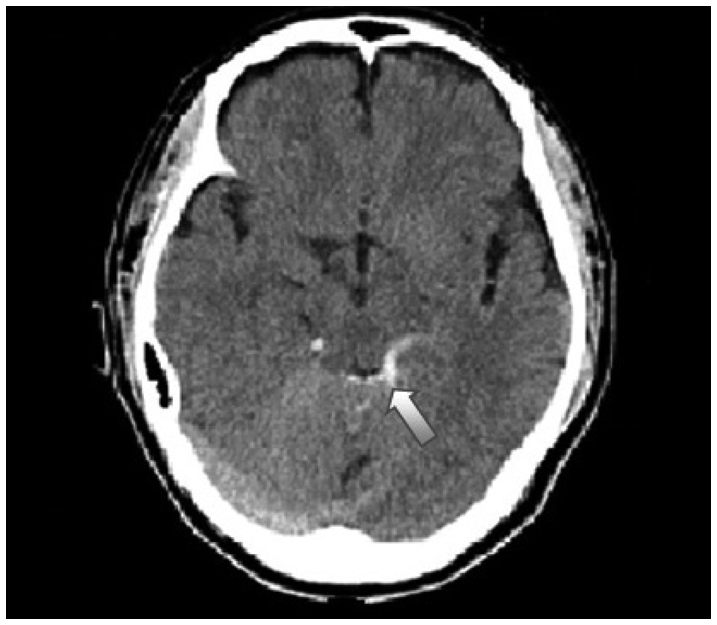
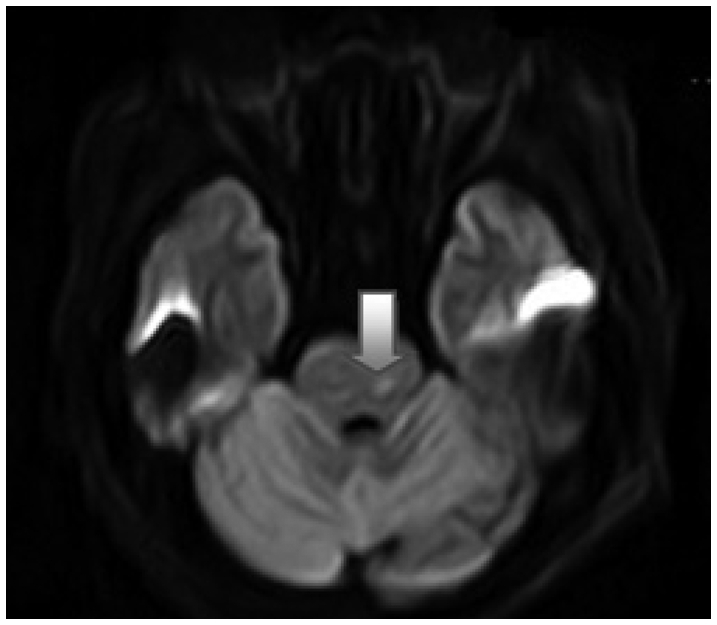
All patients had CT study performed on admission and, in selected cases, MR imaging studies were done to characterize several variables involved in TBSI with special reference to correlation among various parameters on patients' prognosis. Arbitrarily, the clinical parameters were classified as two categories. The first was demographic one such as age and gender. The second was five injury parameters which were mode of injury (high speed, traffic injury and low speed, non-traffic injury), presence of combined injury, impact site on scalp (supratentorial and infratentorial), presence of skull fracture, and lastly the radiologic findings which showed three kinds of pattern : traumatic subarachnoid hemorrhage (TSAH) alone around interpedunculo-ambient cistern (Type 1) (Fig. 1), TSAH with TBSI as a form of diffuse axonal injury (Type 2)(Fig. 2), Type 2 injury associated with supratentorial mass lesions (Type 3). The resultant outcome was conveniently evaluated with two categories; the first was GCS as an early prognosticator and the second was GOS as a late one at the 6 months after trauma.
The age distribution of the 136 TBSI cases showed 96 male (71%) and 40 female (29%), where their mean age was 43.9±21.9 years (range 4-89 years). There was only weak impact of age on GCS by univariate analysis (p<0.054) (Table 3).
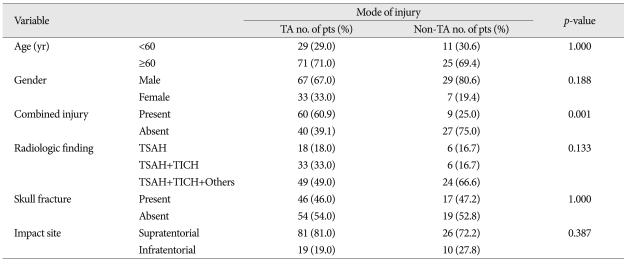
The mode of injury had a significant effect on combined injury, where the high speed, traffic accident had a definite impact on combined injury pattern, compared with low speed, non-traffic accident (p<0.001) (Table 1).
This was mainly assessed from the history and injuries on the scalp, skull, and brain shown on radiologic findings. When we analyzed the effect of scalp impact on brain stem, only two kinds of impact site (supratentorial and infratentorial portion) was selected, because it was considered convenient and easy to evaluate the results of impact shown on radiologic findings. In view of scalp impact, there was a preponderance of supratentorial impact (107 cases, 79%), compared with infratentorial one (29 cases, 21%) (Table 1).
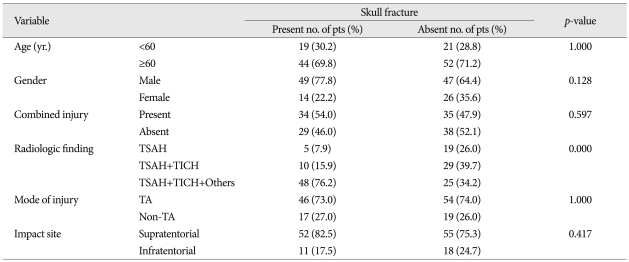
In univariate analysis, the variables such as radiologic findings (p<0.000), mode of injury (p<0.002), impact site on scalp (p<0.052), age (0.054), and skull fracture (p<0.057) had various impact on GCS score, in order of statistical significance (Table 3). The radiologic findings only showed strong correlation to GOS (p<0.001) (Table 4). In multivariate analysis, the individual variables to enhance the unfavorable effect on GCS are radiologic findings [odds ratio (OR) 7.327, 95% confidence interval (CI)], mode of injury (non-traffic injury, OR : 4.499, 95% CI), and age below 60 (OR : 3141) (Table 5).
In univariate analysis, the radiologic findings only showed strong causal relationship to GOS as late stage prognosticator (p<0.000) (Table 4). In multivariate analysis, the parameters to cause worse impact on GOS as a late stage prognosticator were radiologic findings (OR : 25.420, 95% CI), age (OR : 2.674, 95% CI).
Nervous and/or vascular compression against the tentorial notch mostly occurs at its lateral portion due to the shortest distance to the brain stem and near the level of pontomesencephalic junction. These lesions are considered to result from the shearing mechanism in and around the brain stem very close to the tentorial edge. For example, an injury of lower brain stem could be caused by hyperextension of the cervical vertebrae or reciprocal actions of fracture of the clivus and the direct effect on the brain stem by acceleration or rotational forces26). According to TBSI case reports to date, the frequent site of hemorrhage or contusion site is confined to dorsal side of midbrain23), cranial nerves16), whole brain stem26), cerebellum22), combined with upper cervical spinal injuries, clinically presenting as hemiparesis27). In terms of cranial impact site, literature reviews addressed there is an association between occipital blows and primary cerebellar and brain stem lesions6,37). But, another supportive review showed a preponderance of occipital impacts among the cases with primary brain stem lesions which were associated with cerebellar contusion, laceration, and hemorrhage. This clinical evidence was well verified in animal study using fluid percussion injury model under the hypothesis that the cerebellum is susceptible to selective Purkinje cell loss as well as white matter dysfunction22). In addition to that, all impacts to the neck, although few in numbers, was known to give primary brain stem lesions7).
Until Adams et al.1) addressed diffuse brain damage of immediate impact type which strongly correlates DAI to focal lesions in dorsolateral quadrant of the brain stem, there had been so much controversy regarding the existence of primary brain stem injury in isolation without any other pathology in blunt head injury19). The classical DAI typically occurs after head impact and render the victims unconscious at the moment of impact. The DAI is the exact pathological basis, presenting together with or without hemorrhage extending into ventricle, basal ganglia, corpus callosum, and subarachnoid space which are presumably caused by diffuse shearing injury. Generally, the traumatic lesions of the brain stem are classified into two types : primarily, it is caused at the time of impact, and secondarily, associated with supratentorial mass lesions13), and they could be differentiated from secondary brain stem lesions because they are usually observed on the dorsal side of the midbrain. In other words, the primary damage to the brain stem occurs mostly in the tegmentum of brain, more frequently than those lesions in cerebral peduncles or basis pontis. They supposedly result from shearing strains at the craniocervical junction due to fixation by the edge of the tentorium and odontoid peg which played any part on medulla7). Also, it is asserted that the brain stem, hypothalamus and subthalamus be almost always examined for shearing of nerve fibers and small vessels to produce infarcts and hemorrhages6), playing an important role in survival and posttraumatic long-term sequelae29), because the mortality rate for secondary TBSI was two to three times greater than for those with transtentorial hematoma alone due to cranial injuries8).
The TBSI, due to initial trauma of the head, is distinguished from secondary brain stem lesions due to shift and distortion of the brain stem by raised intracranial pressure after injury. Significantly higher incidence of hematoma is in the hemorrhagic cases and the commonest site of hemorrhage is either pons alone or in association with midbrain, thalamus and hypothalamus32). Secondary midbrain lesions are paramedian- often bilateral hemorrhage or necrosis in a distorted midbrain. Whereas, primary lesions are present in an undistorted brain stem and they are lateral, tegmental, and often unilateral microhemorrhages. Another interesting pathology frequently occuring in the supratentorial area is hemispheric injury either in the form of supratentorial or hypothalamo-pituitary area, where the latter type of injury has no correlationship to site of cranial impact29). Andrews et al.3) reported no patient with frontal or parieto-occipital hematoma had clinical signs of transtentorial herniation at admission or subsequently, whereas those with temporal or temporo-parietal lesions had signs of herniation, and no patient with temporal or temporo-parietal hematoma smaller than 30 cc had signs of transtentorial herniation, and appear to be at greater risk of brain stem compression. Therefore, the presence or absence of hematoma affects a great impact on the prognosis, simply causing direct contusion on brain stem and/or secondary herniation into brain stem. Lastly, in dealing with brain stem injury from basal skull fracture and road traffic accident, we must concentrate on the possibility of combined injury on hypothalamic injury29). In terms of skull fracture, cortical laceration and contusion in the cases with primary brain stem lesions, there was no significant difference in the distribution of fractures, but there were more fractures at middle cranial fossa and a preponderance of cerebellar and a less convincing excess of occipital and corpus callosum lesions in patients with primary brain stem lesions7).
Before CT era, midbrain damage as a major DAI site, could be only and easily determined by autopsy, volumetric proton study5), and/or evoked potential study30). The prognosis of patient is dependent on the severity and site of head injury incurred. After CT era, due to its ability to demonstrate the nature, sites, and multiplicity of traumatic brain injury, CT is now the primary diagnostic method for head injury. It is also very useful to elucidate classical DAI and posterior fossa lesions based on direct and indirect signs which include focal hemorrhage, significant contrast enhancement, hemorrhagic contusion, and edema of brain stem33), appearing as areas of high-, mixed-, and low density on the scan. Indirect signs are obliteration of the pontine, cerebello-pontine angle, and perimesencephalic cisterns. Therefore, many cases of TBSI as an indirect evidence were reported where the hematoma were localized along tentorium20), for which Kim et al.17) proposed supratentorial impact site as mostly occipital region, midbrain tegmentum4,24), interpedunculo-ambient cisterns14,21,36), cisterna magna18), and cerebellum22) with or without supratentorial abnormalities. There were many DAI-compatible cases not detected even with CT whose clinical severity could not be evaluated in acute stage. Instead, MRI provides a more sophisticated display of brain stem with improved contrast resolution of structures not appreciated on CT. Therefore, acute stage MRI is used in place of CT or added to CT, because of some limitation of CT in detecting, localizing, and characterizing diffuse injury and posterior fossa lesions5); for example, in differentiating between two patterns of TBSI such as ventral or dorsal location28). Additionally, MRI is more helpful than CT in detecting non-hemorrhagic lesions, cortical contusions, diffuse axonal injury such as supratentorial injury in corpus callosum, and even in normal CT finding when neurological condition could not be explained11,12,15,21,36). Nowadays, electrophysiological study could be added as a more powerful prognostic tool34).
From a prognostic model study for TBSI, age, skull fracture and superimposed mass lesion are the most prognostic factors among the large number of variable tested, where age is the most reliable prognostic variable available at the time of admission. The gender of patient, previous history of hypertension, diabetes mellitus, or alcoholism may also influence the prognosis31). In pediatric cases, the frequency and distribution of TBSI are similar to those of adults35), but the skull fracture is associated with reduced death rate in the younger age group due to dissipated kinetic energy in fracturing the skull, and outcome did not correlate with significantly with morphological patterns of injury or the presence of extracranial injuries8). Generally, poor prognosis is a common feature following severe traumatic brain injury, especially more common in those with TBSI. However, many cases of TBSI following closed head injury were verified and have been increasingly reported with good outcome, especially in those with a single brain stem lesion. TBSI is no more an absolute indicator of poor outcome, because the relationship between TBSI and outcome is still unclear and the types of TBSI are still poorly understood. Therefore, the understanding of anatomy and extent of TBSI, as well as its relationship to supratentorial abnormalities is strongly recommended to estimate actual outcome. The first hypothesis is an anatomical variation in tentorial apertures and their relationship to adjacent structures such as midbrain, cerebellum, and oculomotor nerve which may influence the degree of brain stem distortion in case of acceleration-deceleration injuries2,25). The second hypothesis is that TBSI may occur alone or in association with other cranial injuries. Head injury carries a much graver prognosis when brain stem is involved33). Since severe head injury is often characterized by injury to several sites, both intra-and extra-axial, there may be no clear-cut clinical evidence of a specific brain stem lesion. The most significant lesion may not be suspected until the patient fails to exhibit normal signs of recovery or it may be an unexpected autopsy findings33). Relating the location of the lesions and outcome, the death appeared to be closely linked to the phenomenon of bilateral pontine lesions, especially if bilateral upper pontine lesions are involved. The extent of supratentorial lesions had no bearing on survival in the absence of brain stem lesions10).
The radiologic finding (Type 2 and 3 injury pattern), mode of injury (traffic accident), and age (≥60 yr) were revealed to cause an unfavorable effect on GCS . But, in case of GOS, the radiologic finding and age showed an unfavorable influence. Among them, the radiological finding showed the most strong effect on both of them. Therefore, in cases with TBSI, it is strongly suggested that not only the radiologic finding be assessed carefully as the most important prognosticator but age of victims and mode of injury be evaluated simultaneously as another unfavorable factors.
Acknowledgements
This study was supported by a grant (YUMCM-2008-27) from Wonju College of Medicine.
References
1. Adams H, Mitchell DE, Graham DI, Doyle D. Diffuse brain damage of immediate impact type. Its relationship to 'primary brain-stem damage' in head injury. Brain. 1977; 100:489–502. PMID: 589428.
2. Adler DE, Milhorat TH. The tentorial notch: anatomical variation, morphometric analysis, and classification in 100 human autopsy cases. J Neurosurg. 2002; 96:1103–1112. PMID: 12066913.

3. Andrews BT, Chiles BW 3rd, Olsen WL, Pitts LH. The effect of intracerebral hematoma location on the risk of brain-stem compression and on clinical outcome. J Neurosurg. 1988; 69:518–522. PMID: 3418383.

4. Bouras T, Stranjalis G, Sakas DE. Traumatic midbrain hematoma in a patient presenting with an isolated palsy of voluntary facial movements. Case report. J Neurosurg. 2007; 107:158–160. PMID: 17639886.

5. Carpentier A, Galanaud D, Puybasset L, Muller JC, Lescot T, Boch AL, et al. Early morphologic and spectroscopic magnetic resonance in severe traumatic brain injuries can detect "invisible brain stem damage" and predict "vegetative states". J Neurotrauma. 2006; 23:674–685. PMID: 16689669.

6. Courville CB. Trauma of the Central Nervous System. 1945. Baltimor: Williams and Wilkins;Chap 4.
7. Crompton MR. Brainstem lesions due to closed head injury. Lancet. 1971; 1:669–673. PMID: 4101614.
8. Eder HG, Legat JA, Gruber W. Traumatic brain stem lesions in children. Childs Nerv Syst. 2000; 16:21–24. PMID: 10672425.

9. Firsching R, Woischneck D, Klein S, Ludwig K, Döhring W. Brain stem lesions after head injury. Neurol Res. 2002; 24:145–146. PMID: 11877897.

10. Firsching R, Woischneck D, Klein S, Reissberg S, Döhring W, Peters B. Classification of severe head injury based on magnetic resonance imaging. Acta Neurochir (Wien). 2001; 143:263–271. PMID: 11460914.

11. Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988; 150:673–682. PMID: 3257625.

12. Gentry LR, Godersky JC, Thompson BH. Traumatic brain stem injury : MR imaging. Radiology. 1989; 171:177–187. PMID: 2928523.
13. Hashimoto T, Nakamura N, Richard KE, Frowein RA. Primary brain stem lesions caused by closed head injuries. Neurosurg Rev. 1993; 16:291–298. PMID: 8127442.

14. Hirano A, Matsumura S, Maeda Y, Hashimoto Y, Hirai H. [Two cases of isolated ambient cistern hematoma after head injury]. No To Shinkei. 1995; 47:281–284. PMID: 7669431.
15. Kara A, Celik SE, Dalbayrak S, Yilmaz M, Akansel G, Tireli G. Magnetic resonance imaging finding in severe head injury patients with normal computerized tomography. Turk Neurosurg. 2008; 18:1–9. PMID: 18382970.
16. Katsuno M, Kobayashi S, Yokota H, Teramoto A. [Primary oculomotor nerve palsy due to mild head injury--report of two cases]. Brain Nerve. 2008; 60:89–91. PMID: 18232337.
17. Kim YS, Lim HY, Nah JH, Doh JO, Chang KS. Traumatic tentorial hemorrhage. J Korean Neurosurg Soc. 1986; 15:439–444.
18. Kinoshita Y, Tsuru E, Yasukouchi H, Yokota A. [A case of hematoma in cisterna magna after mild head injury]. No To Shinkei. 2000; 52:320–323. PMID: 10793419.
19. Mitchell DE, Adams JH. Primary focal impact damage to the brainstem in blunt head injuries. Does it exist? Lancet. 1973; 2:215–218. PMID: 4124416.
20. Moskała M, Polak J, Moskała A, Kleinrok K, Zawiliński J. Haematoma of the tentorium cerebelli - new pathology or new prognostic factor in neurotraumatology? A preliminary report. Neurol Neurochir Pol. 2007; 41:234–240. PMID: 17629817.
21. Okuchi K, Fujioka M, Konobu T, Fujikawa A, Nishimura A, Miyamoto S, et al. [Traumatic primary brain stem injury and ambient cistern hematoma evaluated with magnetic resonance imaging]. No Shinkei Geka. 1993; 21:799–804. PMID: 8377896.
22. Park E, Ai J, Baker AJ. Cerebellar injury : clinical relevance and potential in traumatic brain injury research. Prog brain Res. 2007; 161:327–338. PMID: 17618988.
23. Rosenblum WI, Greenberg RP, Seelig JM, Becker DP. Midbrain lesions : frequent and significant prognostic feature in closed head injury. Neurosurgery. 1981; 9:613–620. PMID: 7322325.
24. Saeki N, Otaki M, Oka N, Takase M. [A case of hematoma localized to midbrain tegmentum following closed head injury (author's transl)]. No Shinkei Geka. 1981; 9:1193–1197. PMID: 7290323.
25. Saeki N, Yamaura A, Sunami K. Brain stem contusion due to tentorial coup injury : case report and pathomechanical analysis from normal cadavers. Br J Neurosurg. 1998; 12:151–155. PMID: 11013669.
26. Sato M, Kodama N, Yamaguchi K. Post-traumatic brain stem distortion : a case report. Surg Neurol. 1999; 51:613–616. PMID: 10369228.
27. Se YB, Kim CH, Bak KH, Kim JM. Traumatic brainstem hemorrhage presenting with hemiparesis. J Korean Neurosurg Soc. 2009; 45:176–178. PMID: 19352480.

28. Shibata Y, Matsumura A, Meguro K, Narushima K. Differentiation of mechanism and prognosis of traumatic brain stem lesions detected by magnetic resonance imaging in the acute stage. Clin Neurol Neurosurg. 2000; 102:124–128. PMID: 10996708.

29. Shukla D, Mahadevan A, Sastry KV, Shankar SK. Pathology of post traumatic brainstem and hypothalamic injuries. Clin Neuropathol. 2007; 26:197–209. PMID: 17907596.

30. Soldner F, Hölper BM, Choné L, Wallenfang T. Evoked potentials in acute head injured patients with MRI-detected intracerebral lesions. Acta Neurochir (Wien). 2001; 143:873–883. PMID: 11685619.

31. Stewart WA, Litten SP, Sheehe PR. A prognostic model for brain stem injury. Surg Neurol. 1973; 1:303–310. PMID: 4724952.
32. Tandon PN. Brain stem hemorrhage in cranio-cerebral trauma. Acta Neurol Scand. 1964; 40:375–385. PMID: 14215769.

33. Tsai FY, Teal JS, Quinn MF, Itabashi HH, Huprich JE, Ahmadi J, et al. CT of brainstem injury. AJR Am J Roentgenol. 1980; 134:717–723. PMID: 6767357.

34. Wedekind C, Hesselmann V, Lippert-Grüner M, Ebel M. Trauma to the pontomesencephalic brainstem-a major clue to the prognosis of severe traumatic brain injury. Br J Neurosurg. 2002; 16:256–260. PMID: 12201395.

35. Woischneck D, Klein S, Reissberg S, Peters B, Avenarius S, Günther G, et al. Prognosis of brain stem lesion in children with head injury. Childs Nerv Syst. 2003; 19:174–178. PMID: 12644869.

36. Wong CW. The MRI and CT evidence of primary brain stem injury. Surg Neurol. 1993; 39:37–40. PMID: 8451717.

37. Wright RL. Traumatic hematomas of the posterior cranial fossa. J Neurosurg. 1966; 25:402–409. PMID: 5297035.

Fig. 1
This illutrates Type 1 brain stem injury which has hemorrhage around brain stem (white arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download