Abstract
Parasagittal meningioma often presents as peritumoral brain edema (PTBE). The risk of edema increases when the tumor occludes the superior sagittal sinus (SSS). Although PTBE may be expected based on the patient’s symptoms or radiologic findings, extensive brain swelling and extracranial herniation during elective surgery are rare. Herniation during surgery could lead to irreversible neurological damage and even brain rupture. We report a case of a failed routine craniotomy for a parasagittal meningioma with complete occlusion of the posterior third of the SSS in a 30-year-old male patient. The patient developed extensive brain swelling and extracranial herniation during surgery.
Meningiomas are histologically benign, slow growing, and of extracerebral origin. Peritumoral brain edema (PTBE) accompanies 40–60% of meningiomas [1]. Several causative factors have been proposed, including tumor size, location, histologic differentiation, vascularity, venous stasis, arterial blood supply, and the presence of vascular endothelial growth factors (VEGFs) [234]. With extensive PTBE, brain herniation could occur during surgery, leading to irreversible neurological damage, even brain rupture [5].
Here, we introduce a case of a parasagittal meningioma along the posterior third of the superior sagittal sinus (SSS) with total occlusion and extensive edema in a 30-year-old male patient. The resection was conducted in two steps due to severe intraoperative brain swelling during the initial attempt to excise the tumor.
A 30-year-old male presented with headaches and visual disturbances for 1 year. He had left homonymous inferior quadranopsia. Brain magnetic resonance imaging showed a 46×30×37 mm dural-based enhancing mass in both parietal convexities involving the SSS with intermediate signal intensity on T1WI and T2WI (Fig. 1). On digital subtraction angiography, the hypervascular tumor was found to be supplied mainly by the terminal branch of the right middle meningeal artery, and total occlusion of the posterior third of the SSS was confirmed during the venous phase (Fig. 1). Preoperative embolization of the right middle meningeal artery was conducted 2 days before the surgery.
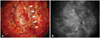
On the day of the surgery, the patient was placed in the left lateral position with head elevated. A 10×15 cm sized left parieto-occipital craniotomy crossing the midline was performed. After the bone flap was removed, indocyanine green (ICG) dye was injected to determine the location of the tumor, sinuses, and cortical veins. Doppler-evaluated sinus flow was absent (Fig. 2). SSS-based durotomy was conducted. A well-demarcated, brownish mass was strongly attached to the dura mater.
Brain swelling and strangulation was observed immediately post-durotomy. We found no etiologies, such as hypercarbia, hypoxia, overhydration, or faulty positioning of the head, which would lead to impaired cerebral venous drainage. Infusion of 200 cc of 20% mannitol was ineffective. Hence, we decided not to remove the tumor and instead performed an artificial duroplasty without bone flap re-fixation. An intracranial pressure (ICP) monitor was also inserted. In the intensive care unit, the patient’s ICP was between 9–14 mm Hg, and the patient recovered from anesthesia.
One month later, the second surgery was conducted. We used the same incision, but the craniotomy was performed more laterally (15×15 cm). SSS-based right-side durotomy was performed, and the tumor was dissected from the normal brain tissue on the lateral side. Edema was persistent, but extracranial herniation or strangulation was not seen. The SSS border was also dissected, and its feeding vessel was coagulated. The left-side tumor was removed in the same manner. The proximal and distal borders of the SSS attached to the tumor were tied with 5–0 silk and cut using a blade. The tumor had filled the inferior SSS tightly and pathologic diagnosis was a meningioma (World Health Organization grade I). The persistent slight edema after tumor removal required outpatient observation until the brain swelling was reduced, and cranioplasty was performed after the confirmation of soft sensation at the bone defect site. Currently, at 24 months post-surgery, the patient is doing very well with no complications (Fig. 3).
Our patient had worsening neurological symptoms, including headaches and visual disturbances, which were indications for surgical intervention. Also, considering the patient’s young age, tumor size, and lack of calcification, the tumor was more likely to grow quickly.
Sindou et al. [6] reported that tumor invasion of the major dural sinuses, especially meningiomas, present the surgeon with the dilemma of whether to 1) leave the fragment invading the sinus, thus creating a higher risk of recurrence or 2) attempt total removal with or without venous reconstruction and expose the patient to a potentially greater operative danger. Hence, during the preoperative evaluation, it is important to assess 1) the degree of sinus occlusion, 2) the anatomy of the sinus and associated large cortical draining veins, and 3) the development of venous collaterals [78]. In lesions of the SSS anterior third, total removal can usually be done, including removal of the sagittal sinus and falx, even if the sinus is open. In the middle or posterior thirds, total removal can be done if the sinus is occluded. If only the edge of the sinus is involved, the sinus may be opened to remove the residual tumor plaque and edge of the sinus wall; the opening is then progressively closed with a continuous suture. When the tumor has extensively invaded the middle or posterior third of the SSS and the sinus is still open, the tumor must be left in the wall [9]. In our case, the posterior third of the SSS was totally occluded, and we found no particularly important draining veins on preoperative digital subtraction angiography and intraoperative ICG findings. Also, we found no invasion of cortical veins during the first operation.
In addition to venous status, there are many factors to consider before surgery to predict PTBE. Short duration of symptoms [10], large tumor volume [11], and high brain edema index [(the volume of the tumor plus PTBE)/the volume of the tumor] [12] are associated with PTBE. In addition, the types of blood supply and presence of VEGFs have recently been implicated as causative factors of PTBE [3]. In our case, there were no acute symptoms, but tumor size was 13 mL, brain edema index was 15.6 (higher group is ≥10, lower group is <10), and pial blush was present. Given the presence of these risk factors, brain swelling during the operation was expected. Thus, we considered ways to minimize cerebral edema intra-operatively, such as correct positioning during the operation, proper anesthesiologic management, and large craniotomy size. However, brain swelling was greater than anticipated. Therefore, the operative procedure was abandoned, and medical treatments were used to control ICP.
Prone positioning during craniotomy could increase intraabdominal pressure and compromise venous return, which could lead to cerebral venous hypertension and increased ICP [13]. Accordingly, we positioned the patient in the supine lateral position with a large cushion under the ipsilateral shoulder, which facilitated head rotation; we then turned the neck, being careful not to press on the jugular vein. The patient’s neck was long, flexible, and shoulder was narrow. So, there was no need to take pose the park-bench position.
Profound brain swelling and herniation during elective craniotomy related to either hypercarbia or high venous pressure was common before modern neuroanesthesia. Such an event is now rare and occasionally found after evacuation of a post-traumatic acute subdural hematoma [5]. Some authors postulate that cerebral hyperemia and acute ICP changes occur directly as a result of either acute subarachnoid hemorrhage (SAH) or intraventricular hemorrhage (IVH), or indirectly by various neurovascular reflex mechanisms induced by SAH during elective craniotomy [1415]. However, SAH or IVH were not observed on computed tomography scan in our case.
According to one study, brain herniation occurs due to blockage of the veins and arteries, caused by shearing and compressive forces between the dural edge and brain tissue. Venous congestion induces further edema in the protruding parts of the brain, thus causing a lesion by strangulated necrosis and hypoxia. Therefore, Csókay et al. [16] recommend stellate-manner durotomy. In this case, we performed a small semicircular-shaped durotomy; thus, we believe the shape and size of durotomy may be a factor in extracranial herniation.
While our patient had some risk factors for cerebral edema, they were not severe enough to explain the extracranial herniation or brain strangulation we observed, as compared to other craniotomy cases. Hence, even in elective surgery, we propose meticulous evaluation for PTBE before surgery, and a sufficiently large craniotomy and durotomy exposing peritumoral T2 high signal intensity lesions, if brain swelling or herniation is at all anticipated.
In conclusion, because of significant PTBE during our initial attempt to resect a meningioma, we resected the parasagittal meningioma along the posterior third of the SSS with total occlusion in two stages. Even in elective surgery, meticulous evaluation for the possibility of PTBE is required before surgery. Furthermore, a sufficiently large craniotomy and durotomy exposing peritumoral T2 high signal intensity lesions should be performed despite the slightly higher risk of cerebral edema.
Figures and Tables
Fig. 1
Preoperative magnetic resonance imaging and cerebral angiographic findings. T2-weighted axial image shows dural-based iso-signal intensity mass in the parietal convexity along the superior sagittal sinus (SSS) with extensive perilesional edema (A); gadolinium-enhanced axial and sagittal image shows homogenous enhancement pattern (B and C); and right internal carotid angiography shows total occlusion of posterior third of the SSS (D).
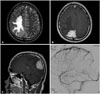
Fig. 2
Transdural observation of the cortical arteries, veins, and venous sinus was performed using indocyanine green (ICG) videography. Visualization of the dural attachment of meningioma enabled safe and appropriate dural opening, minimizing damage to the venous channels. General microscopic findings (A) and ICG videographic findings (B). The black open arrows mark the tumor margin, and the white closed arrows mark the right lateral border of posterior third of the superior sagittal sinus.
References
1. Abe T, Black PM, Ojemann RG, Hedley-White ET. Cerebral edema in intracranial meningiomas: evidence for local and diffuse patterns and factors associated with its occurrence. Surg Neurol. 1994; 42:471–475.

2. Challa VR, Moody DM, Marshall RB, Kelly DL Jr. The vascular component in meningiomas associated with severe cerebral edema. Neurosurgery. 1980; 7:363–368.

3. Go KG, Wilmink JT, Molenaar WM. Peritumoral brain edema associated with meningiomas. Neurosurgery. 1988; 23:175–179.

4. Inamura T, Nishio S, Takeshita I, Fujiwara S, Fukui M. Peritumoral brain edema in meningiomas--influence of vascular supply on its development. Neurosurgery. 1992; 31:179–185.

5. Whittle IR, Viswanathan R. Acute intraoperative brain herniation during elective neurosurgery: pathophysiology and management considerations. J Neurol Neurosurg Psychiatry. 1996; 61:584–590.

6. Sindou M, Auque J, Jouanneau E. Neurosurgery and the intracranial venous system. Acta Neurochir Suppl. 2005; 94:167–175.

7. Mantovani A, Di Maio S, Ferreira MJ, Sekhar LN. Management of meningiomas invading the major dural venous sinuses: operative technique, results, and potential benefit for higher grade tumors. World Neurosurg. 2014; 82:455–467.

8. Oh IH, Park BJ, Choi SK, Lim YJ. Transient neurologic deterioration after total removal of parasagittal meningioma including completely occluding superior sagittal sinus. J Korean Neurosurg Soc. 2009; 46:71–73.

9. Ojemann RG. Management of cranial and spinal meningiomas (honored guest presentation). Clin Neurosurg. 1993; 40:321–383.
10. Stevens JM, Ruiz JS, Kendall BE. Observations on peritumoral oedema in meningioma. Part II: mechanisms of oedema production. Neuroradiology. 1983; 25:125–131.
11. Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999; 85:936–944.
12. Osawa T, Tosaka M, Nagaishi M, Yoshimoto Y. Factors affecting peritumoral brain edema in meningioma: special histological subtypes with prominently extensive edema. J Neurooncol. 2013; 111:49–57.

13. Roth C, Ferbert A, Deinsberger W, et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014; 21:186–191.

14. Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988; 22:654–661.

15. Nornes H. The role of intracranial pressure in the arrest of hemorrhage in patients with ruptured intracranial aneurysm. J Neurosurg. 1973; 39:226–234.

16. Csókay A, Nagy L, Vimláti L Jr. [Vascular tunnel creation to improve the effect of decompressive craniectomy in severe traumatic cerebral edema]. Orv Hetil. 2001; 142:75–78.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download