Abstract
This case report presents a fully thrombosed large aneurysm of middle cerebral artery mimicking an intra-axial brain tumor in a 54-year-old male patient. A small mass like lesion was found incidentally in right frontal area. Brain magnetic resonance image showed dark signal intensity on T2-weighted images and peripheral high signal intensity on T1-weighted images with peripheral rim enhancement. We considered intra-axial tumors such as glioma or metastatic tumor as a differential diagnosis. The lesion was approached transcortically, and intraoperatively, the lesion was found to be a large thrombosed aneurysm originating from the lateral lenticulostriate artery of right middle cerebral artery. One vascular clip was applied at the parent artery, and the thrombosed aneurysm was totally removed. There have been many reports of other intracranial lesions wrongly diagnosed as intracranial neoplasms. And thrombosed aneurysms mimicking intracranial neoplasm have been reported in 4 cases previously. According to those case reports, there were no efficient imaging tools to differentiate between these thrombosed aneurysms and intracranial neoplasms. We reviewed those reports and considered about the efficient method to diagnosed accurately before surgery. To sum up, when a patient presents with an intracranial lesion lying on the course of major or distal cerebral arteries, the surgeon should have thrombosed aneurysm in mind as one of the differential diagnosis and be prepared when surgically treating such lesions.
Approximately 14% to 20% of all intracranial aneurysms originate along middle cerebral artery (MCA) [1]. Among those MCA aneurysms, aneurysms of the M1 segment are second in frequency to bifurcation aneurysms. Aneurysms at proximal M1 segment represent 2% to 12% of all MCA aneurysms [2]. They are composed of lenticulostriate artery or anterior temporal artery saccular aneurysm.
Spontaneous thrombosis of a saccular aneurysm is known to occur, and occurs in about 40% of giant aneurysms. It may be symptomatic secondary to mass effect or stroke. However, spontaneous thrombosis of non-giant (<25 mm) saccular aneurysm is much less identified [3].
We report a case of fully thrombosed non-giant aneurysm which mimics intra-axial tumor with peripheral rim enhancement in magnetic resonance image (MRI). In addition, we reviewed the literatures of thrombosed aneurysm which mimics intracranial tumor.
A 54-year-old male patient, with a history of traffic accident a week ago, presented to the hospital with sustained headache after the accident. 7 years ago, he had taken the brain computed tomography (CT), and small mass like lesion was found in right frontal area (Fig. 1). He was lost to follow-up since then. The CT scan taken this time showed enlargement of the mass-like lesion with surrounding edema (Fig. 1). To evaluated the mass, brain MRI was taken. The MRI showed dark signal intensity on T2-weighted images and peripheral high signal intensity on T1-weighted images with peripheral rim enhancement (Fig. 2). Additional studies such as diffusion-weighted MRI, perfusion MRI, magnetic resonance spectroscopy or angiography were not conducted considering poor financial status of the patient. Differential diagnosis included intra-axial tumors such as glioma, metastatic tumor or ganglioglioma.
We performed the operation using a right pterional craniotomy under general anesthesia. Navigation-guided transcortical approach was performed, and the mass was exposed (Fig. 3). The lesion was found to be a large thrombosed aneurysm originating from the M1 segment of right middle cerebral artery. We applied one vascular clip at the parent artery and the distal end was coagulated by bipolar coagulator. After cutting the parent artery distal to the vascular clip, thrombosed aneurysm was totally removed (Fig. 3). Pathologic examination confirmed the diagnosis of thrombosed aneurysm. It showed aneurysmal sac with organizing thrombi and atherosclerotic change (Fig. 4). Postoperative images showed total removal of the lesion (Fig. 5). Postoperative angiogram was done to confirm the vascular clip applied to the proximal right middle cerebral artery and to rule out other abnormal vascular lesions (Fig. 6). The patient recovered well and discharged a week later.
Intracranial aneurysms are common and affecting approximately 5% of the population [4]. And MCA aneurysms represent approximately one third of intracranial aneurysm [5]. Furthermore, aneurysms of the M1 segment represent 2% to 12% of all MCA aneurysms [2]. Proximal MCA aneurysms can be further divided into superior wall type and inferior wall type [6]. Superior wall type of M1 segment aneurysms arise at the origins of the lenticulostriate arteries and project into the frontal lobe. Inferior wall type aneurysms arise at the origin of the anterior temporal artery or the temporopolar artery and project toward the temporal lobe in an anterolateral projection. This case belongs to the former subtype, superior wall type of M1 segment aneurysm. The thrombosed aneurysm was projecting superiorly into the frontal area. The aneurysm of this case can be classified as a lenticulostriate artery aneurysm. Lenticulostriate artery aneurysms are rare entities, especially rare in patients without underlying comorbidities such as moyamoya disease, mycotic aneurysms, other vasculopathies, or hypertension or drug use in adults [7]. In addition, when they have been reported, they are typically associated with hemorrhage [7].
Giant aneurysms, which are bigger than 2.5 cm in diameter, are unusual, representing 5-13% of all intracranial aneurysm [8]. These giant aneurysms usually present as masses rather than hemorrhages. In addition, spontaneous thrombosis of an intracranial aneurysm is well known usually in giant aneurysms [9]. Black and German [10] demonstrated the relationship of aneurysmal volume and aneurysmal orifice to intraaneurysmal thrombus formation by biophysical and hemodynamic studies. A larger ratio of volume to orifice causes slower flow and longer blood retention time, resulting in intraaneurysmal thrombosis. This is the reason why giant intracranial aneurysms have a high incidence of thrombus formation compared to common size aneurysms. However, in this case, thrombosed aneurysm was 1.9 cm in diameter which is defined as "large" aneurysm rather than "giant" aneurysm. And it was even smaller when it was first detected. In this case, location and size of the mass was not easy to consider thrombosed aneurysm as a differential diagnosis. So, this case shows that surgeons should consider thrombosed "large" aneurysm as a differential diagnosis when the size and location of mass is not typical as other giant aneurysms or other thrombosed aneurysms.
There have been many reports of other intracranial lesions wrongly diagnosed as intracranial neoplasms. For example, tuberculoma, which is tuberculosis of the central nervous system (CNS), has been a lot reported up to now [11]. CNS tuberculomas have the ability to mimic a brain neoplasm and can develop in any region of the CNS. It is the result of hematogenous spread from a primary focus in the lung [12]. In clinical field, it is not so rare to misdiagnosed thrombosed aneurysm as a brain tumor, however, thrombosed aneurysms mimicking intracranial neoplasm have been reported only in 4 cases previously (Table 1) [13,14,15,16]. It might be because it seems not so valuable to report. But, in this case, it seemed valuable to share, because as mentioned above, it was not so big as giant aneurysms and the location of aneurysm was lenticulostriate artery which is rare. Among the other reported cases, the aneurysms of 2 cases [13,16] were included in a category of giant aneurysm and the aneurysms of 3 cases [13,14,16] were located in posterior circulation. Every patient of the reported cases was managed successfully by controlling the parent arteries and totally removing the thrombosed aneurysm, and the outcomes were excellent. There were no efficient imaging tools to differentiate between these thrombosed aneurysms and intracranial neoplasms. Conventional angiography and diffusion restriction MRI were not sufficient for differential diagnosis [13,14,15,16]. In our case, both studies were not done because of poor financial status of the patient. However, as the aneurysm was fully thrombosed, both studies would not be useful for diagnosis in this case. Therefore, when a patient presents with an intracranial lesion lying on the course of major cerebral arteries or even on the course of distal cerebral arteries, it is important to have thrombosed aneurysm in mind as one of the differential diagnosis. Though full studies of MRI and conventional angiography are recommended in suspected cases, the clinician must be aware of the limitations of imaging studies in identifying thrombosed aneurysms. Furthermore, in these cases of thrombosed aneurysm, though it has low possibility of rupture because it is fully thrombosed, the surgeon should control the parent artery completely by clipping or trapping to prevent intraoperative or postoperative arterial bleeding.
In conclusion, we report a rare case of thrombosed large aneurysm mimicking intra-axial brain tumor. Peripheral rim enhancement with surrounding edema in MRI can be easily diagnosed as a brain tumor. However, when the lesion is lying on the course of major or distal cerebral arteries, it is important to have thrombosed aneurysm in mind as one of the differential diagnosis and be prepared when surgically treating such lesions.
Figures and Tables
Fig. 1
Computed tomography (CT) scan of brain. A: Well-defined mass showing high density, sized 11 mm in diameter, located in right frontal area is observed in initial CT, 7 years ago. B: Recent CT scan shows enlargement of the mass-like lesion which was noted previously, sized in 19×15 mm, with surrounding edema.
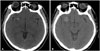
Fig. 2
Magnetic resonance images (MRI) of brain. A: T2-weighted axial MRI. B: Fluid-attenuated inversion recovery (FLAIR) axial MRI. C: Gadolinium enhanced T1-weighted coronal MRI. D: Non-enhanced T1-weighted axial MRI. E: Gadolinium-enhanced T1-weighted axial MRI. F: Gadolinium-enhanced T1-weighted sagittal MRI. A round mass with surrounding edema is noted in right frontal gray matter. It shows peripheral rim enhancement. Dark signal intensity on T2-weighted image (A) and FLAIR image (B) and peripheral high signal intensity on T1-weighted image (D) suggests hemangioma as a differential diagnosis to rule out.
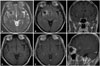
Fig. 3
Intraoperative photographs showing a large thrombosed aneurysm. A: Yellowish round mass (black arrow) was observed after navigation-guided transcortical approach at right frontal area. B: Small artery (white arrow) connected to the mass revealed under the mass. It seemed to be one of the lenticulostriate arteries. C: Vascular clip was applied at the parent artery and the distal end (white arrow) was coagulated by bipolar coagulator. D: The mass was totally excised, and there was no other complication at the operation site.
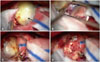
Fig. 4
Gross images of the removed mass. A: Gross image of the mass shows yellowish slithery surface as a vessel wall. B: Cross section of the mass shows thrombus material fully embeded in the aneurysm.
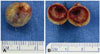
Fig. 5
Postoperative images. A: CT scan shows postoperative changes with vascular clip noted in right sylvian fissure. B: In the MRI, the mass is totally removed and vascular clip as applied parallel to the M1 segment of right middle cerebral artery. There was no other surgical complications.
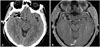
Fig. 6
Right internal carotid angiogram (A) and three-dimensional reconstructed image (B) shows vascular clip applied parallel to the M1 segment of right middle cerebral artery and complete removal of thrombosed aneurysm. The arrow indicates the vascular clip applied site. There was no other vascular abnormalities.

Table 1
Reported cases of thrombosed aneurysm mimicking intracranial tumor

| Author (year) | Sex/age | Location of aneurysm | Size of aneurysm (cm) | Treatment | Misdiagnosis | Outcome |
|---|---|---|---|---|---|---|
| Fifi et al. [15] (2010) | F/37 | Anterior cerebral artery (A2 segment) | 1 | Trapping+aneurysmal sac removal | Dermoid cyst | Good |
| Päsler et al. [14] (2011) | M/22 | Anterior inferior cerebellar artery | N/A | Thrombectomy+reconstruction | Vestibular schwannoma | Good |
| Lan et al. [16] (2012) | M/9 | Posterior inferior cerebellar artery | 4.5 | Clipping+aneurysmal sac removal | Epidermoid cyst | Good |
| Woo et al. [13] (2014) | F/79 | Posterior inferior cerebellar artery | 3.0 | Clipping+aneurysmal sac removal | Infratentorial ependymoma | Good |
| Present case (2014) | M/54 | Middle cerebral artery (M1 segment) | 1.5 | Clipping+aneurysmal sac removal | Intraaxial tumor (such as glioma) | Good |
References
1. Heros RC, Fritsch MJ. Surgical management of middle cerebral artery aneurysms. Neurosurgery. 2001; 48:780–785.

2. Rinne J, Hernesniemi J, Niskanen M, Vapalahti M. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996; 38:2–11.

3. Brownlee RD, Tranmer BI, Sevick RJ, Karmy G, Curry BJ. Spontaneous thrombosis of an unruptured anterior communicating artery aneurysm. An unusual cause of ischemic stroke. Stroke. 1995; 26:1945–1949.

4. Jellinger K. Pathology of intracerebral hemorrhage. Zentralbl Neurochir. 1977; 38:29–42.
5. Wiebers DO, Whisnant JP, Huston J 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 362:103–110.

6. Hosoda K, Fujita S, Kawaguchi T, Shose Y, Hamano S. Saccular aneurysms of the proximal (M1) segment of the middle cerebral artery. Neurosurgery. 1995; 36:441–446.

7. Gandhi CD, Gilad R, Patel AB, Haridas A, Bederson JB. Treatment of ruptured lenticulostriate artery aneurysms. J Neurosurg. 2008; 109:28–37.

8. Morley TP, Barr HW. Giant intracranial aneurysms: diagnosis, course, and management. Clin Neurosurg. 1969; 16:73–94.

9. Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982; 45:1040–1047.

10. Black SP, German WJ. Observations on the relationship between the volume and the size of the orifice of experimental aneurysms. J Neurosurg. 1960; 17:984–990.

11. Jaimovich SG, Thea VC, Guevara M, Gardella JL. Cavernous sinus tuberculoma mimicking a neoplasm: case report, literature review, and diagnostic and treatment suggestions for tuberculomas in rare locations. Surg Neurol Int. 2013; 4:158.

12. Grayeli AB, Redondo A, Salama J, Rey A. Tuberculoma of the cavernous sinus: case report. Neurosurgery. 1998; 42:179–181.

13. Woo PY, Ko NM, Chan KY. Thrombosed large distal posterior inferior cerebellar artery aneurysm mimicking an infratentorial ependymoma. Case Rep Neurol Med. 2014; 2014:435953.

14. Päsler D, Baldauf J, Runge U, Schroeder HW. Intrameatal thrombosed anterior inferior cerebellar artery aneurysm mimicking a vestibular schwannoma. J Neurosurg. 2011; 114:1057–1060.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download