Abstract
Purpose
This study was done to compare the prophylactic efficacy of surfactant with Curosurf® and Newfactan®.
Methods
Preterm infants treated with pulmonary surfactant within 30 minutes after birth from January 2008 to December 2014 were included in this study. The subjects were divided into a group that received Curosurf® (n=163) and a group that received Newfactan® (n=120). The data were collected retrospectively using the patients' medical records.
Results
Demographic factors did not differ between the groups. The incidence rates of surfactant re-dosing, pulmonary air leaks, postnatal steroid therapy use, pulmonary hemorrhage, moderate to severe bronchopulmonary dysplasia, and duration of mechanical ventilation, were not different between the groups. The sequential changes of ventilator parameters after surfactant instillation were not different between the groups. No differences in the incidence rates of patent ductus arteriosus, intraventricular hemorrhage (≥grade III), periventricular leukomalacia, retinopathy of prematurity, necrotizing enterocolitis (≥stage IIa), mortality, sepsis, or duration of hospital stay were observed between the groups.
Respiratory distress syndrome (RDS) is caused by a deficiency in pulmonary surfactant in neonates and is the most common pulmonary morbidity in preterm infants. Exogenous surfactant is an effective treatment for neonatal RDS that reduces the risk of pneumothorax and mortality in neonates.12 Two methods, which are divided into early prophylactic and rescue therapy, have been used to treat RDS based on the time of surfactant therapy in neonates.34 Early prophylactic use of surfactant is defined as surfactant instillation within 10 to 30 min of birth before diagnosis of RDS in infants born at <30-32 weeks of gestation. Rescue therapy corresponds to application of surfactant within the first 12 hrs after birth with clinically confirmed RDS.5 Early prophylactic use of surfactant has been more effective in improving clinical outcomes including mortality in meta-analyses.14678
Since Fujiwara et al.9 successfully applied surfactant in newborn infants in the 1980s, several artificial pulmonary surfactants have been used worldwide to treat RDS of neonates. Several surfactants, such as Surfacten® (Surfactant-TA; Mitsubishi-Tokyo Pharma Corp., Osaka, Japan), Newfactan® (YY-38; Yuhan Pharm Corp., Seoul, Korea), Curosurf® (Poractant alfa; Chiesi Farmaceutici SpA, Parma, Italy), and Infasurf® (Calfactant; ONY Inc., Amherst, NY, USA) have been used in Korea.
Several studies have been conducted in Korea about surfactant therapy for RDS in neonates.101112131415 However, those studies had limitations in study design to compare the efficacy of specific drugs during early prophylactic use in very preterm infants. Therefore, this study was performed to compare the efficacy of early prophylactic use of Curosurf® versus Newfactan® for RDS in infants born at <30 weeks of gestation or with birth weight ≤1,250 g.
Of the 322 infants born at <30 weeks of gestation or with birth weight ≤1,250 g at Ajou University Hospital, Suwon, Korea from January 2008 to December 2014, 283 who were treated within 30 minutes after birth as early prophylactic use of surfactant, were finally enrolled. Of the 39 excluded infants, 13 were not treated with pulmonary surfactant, 22 were treated with surfactant >30 minutes after birth [Curosurf® (n=14) and Newfactan® (n=8)], and four cases were excluded due to life threatening congenital malformations, such as chromosomal abnormalities or cardiac anomalies.
We divided our patients into two groups according to the type of surfactant; 120 infants were treated with Newfactan® from January 2008 to December 2010, and 163 infants were treated with Curosurf® from January 2011 to December 2014. This study protocol was approved by the Institutional Review Board at the Ajou University Hospital.
The data were collected retrospectively from the patients' medical records. Pulmonary surfactant instillation was used as an early prophylactic therapy. Surfactant was administered within 30 min of birth in infants born at <30 weeks of gestation or with birth weight ≤1,250 g in the delivery room or neonatal intensive care unit without radiological evidence of RDS. Curosurf® was administered at 2.5 mL/kg (200 mg/kg) into the trachea via an endotracheal tube using an orogastric tube. Newfactan® was prepared in 4 mL of normal saline and instilled at 4 mL/kg (120 mg/kg) at room temperature without bubbles. All infants in this study were treated with mechanical ventilation after surfactant administration. And, they were extubated as soon as fraction of inspired oxygen (FiO2) <0.3 and mean airway pressure (MAP) <7 cmH2O was reached. Initial administered surfactant was reused when surfactant re-dosing was needed.
We compared the clinical characteristics between the two groups, including gestational age, birth weight, sex, Apgar score, antenatal corticosteroid use, gestational diabetes mellitus, maternal pregnancy-induced hypertension, pathologic chorioamnionitis, and the time of surfactant therapy after birth. We collected the following data associated with RDS outcomes, such as surfactant re-dosing, pulmonary air leaks, duration of mechanical ventilation, duration of invasive ventilation, postnatal steroid therapy, pulmonary hemorrhage, and bronchopulmonary dysplasia (BPD). We also compared complications, including patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), hospital stay, mortality, and sepsis. Indicators of the clinical respiratory status associated with RDS were estimated with the arterial-alveolar oxygen ratio (a/APO2=arterial partial pressure of oxygen (PaO2)/[713×FiO2–PaCO2/0.8]), ventilatory index (VI=MAP×FiO2/PaO2), and MAP. Pulmonary air leaks included pneumothorax, pneumomediastinum, and pulmonary interstitial emphysema.
BPD was defined as requiring supplemental oxygen for at least 28 days after birth and severity was categorized according to the respiratory support required at 36 weeks of postmenstrual age or discharge, whichever came first.16 NEC was defined as modified Bell's criteria ≥stage IIa.17 Grade III or IV IVH and cystic PVL on cranial ultrasound were based upon the Papile grading system.18 Sepsis was defined according to the Centers for Disease Control and Prevention/National Nosocomial Infections Surveillance system definition.19 PDA was diagnosed by color-flow Doppler echocardiography with the presence of two of the following five signs: (1) systolic or continuous murmur, (2) bounding pulse or hyperactive precordial pulse, (3) difficulty maintaining blood pressure, (4) worsening ventilator status, and (5) chest radiographic evidence, i.e. pulmonary congestion or cardiomegaly (a cardiothoracic ratio >60%) with increased pulmonary flow.20
SPSS ver. 21.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The chi-square or Fisher's exact test was used to compare categorical variables, and Student's t-test and the Mann-Whitney U-test were used to compare continuous variables. Repeated-measures analysis of variance was used to compare the respiratory parameters. Categorical data are presented as percentages (%), and continuous data are presented as mean±standard deviation. A P-value <0.05 was considered significant.
Of the 283 newborn infants, 163 were given Curosurf® and 120 were given Newfactan®. No differences in gestational age, birth weight, sex, Apgar scores at 1 and 5 min, antenatal corticosteroid use, or time of surfactant therapy after birth were detected between the groups. Maternal factors, such as gestational diabetes mellitus, pregnancy-induced hypertension, and pathologic chorioamnionitis also showed no difference (Table 1).
Table 2 shows the clinical outcomes associated with RDS. The incidence rates of surfactant re-dosing, pulmonary air leaks, postnatal steroid therapy use, pulmonary hemorrhage, and moderate to severe BPD were not different between the groups. Total duration of mechanical ventilation and duration of invasive ventilation were also not different between the two groups.
We obtained FiO2, MAP, a/APO2, and ventilatory index values at 0, 1, 2, 6, 12, 24, 48, 72, and 96 h after surfactant instillation. The time course of FiO2 showed no significant differences between the two groups (P=0.784) (Fig. 1A). The MAP time course was not different between the two groups (P=0.392) (Fig. 1B). The a/APO2 time course showed no significant statistical differences between the two groups (P=0.621) (Fig. 1C). And, no differences in sequential changes of the ventilatory index were observed over the 96 h after surfactant instillation between the two groups (P=0.924) (Fig. 1D).
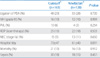
No difference in the incidence rates of PDA ligation, IVH, PVL, ROP, NEC, hospital stay, mortality, or sepsis were detected between the two groups (Table 3).
In this study, we compared the efficacy of Curosurf® and Newfactan®, used as early prophylactic surfactant therapy for RDS. Our data show no significant differences in complication, mortality, or clinical outcomes between Curosurf® and Newfactan®.
Several meta-analyses have reported that early prophylactic surfactant therapy is superior to late selective surfactant therapy. Jobe reported that the prophylactic group had a 50% lower mortality rate compared with that of an untreated group.1 Egberts et al. reported that prophylactic surfactant therapy decreases the incidence rates of severe RDS, mortality, and BPD in preterm infants.21 Soll and Morley found a beneficial effect of prophylactic surfactant therapy in preterm infants born at <30 weeks gestation.8 Kim et al. also reported a significant decrease in the rates of PDA, BPD, and mortality with use of early prophylactic surfactant therapy.12
Several studies have compared the efficacy of pulmonary surfactants used for infants with RDS. Ramanathan, et al. conducted a multicenter study with a large cohort of infants, comparing Curosurf® and Survanta® (Ross Laboratories, Columbus, OH, USA).22 The infants were born at <35 weeks gestation and were administered pulmonary surfactant within 6 h after birth. The authors concluded that Curosurf®, a porcine lung-derived surfactant, decreased mortality rates compared to that of Survanta®. Subjects who received an initial 200 mg/kg Curosurf® dose rapidly reduced their need for supplemental oxygen with fewer additional surfactant doses versus those treated with a 100 mg/kg initial dose. Fujii et al. also showed that Curosurf® is associated with early extubation time and low MAP requirement to maintain adequate oxygenation compared to those of Survanta®.23 However, that study had a relatively smaller sample size and long-term outcomes were not included. Trembath et al. conducted a comparative multicenter study of Curosurf®, Survanta®, and Infasurf® with a large number of infants born at <37 weeks gestation.24 All patients treated with surfactants were enrolled, without considering instillation time. As results, no differences in clinical outcomes, such as BPD, NEC, IVH, or mortality were observed among infants treated with Curosurf®, Survanta®, and Infasurf®.
In Korea, Hong et al. conducted a comparative study of surfactants and reported no difference in the clinical effects between Curosurf® and Newfactan®.25 However, that study had a relatively small sample size and surfactant instillation time was not considered. Additionally, infants born at <36 weeks gestation were all included. Kim et al. conducted a retrospective comparative study in 2013 of 41 infants who received Curosurf® or Surfacten® and instillation time was different between each group.13 No differences in clinical outcomes, such as NEC, IVH, PVL, or ROP, were observed between the Curosurf® and Surfacten® groups. Jeon et al. reported that Infasurf® is equally effective as Surfacten® and Curosurf®.11 They enrolled 332 infants at 24-31 weeks gestation, the infants were administered the surfactant as rescue therapy until 2010 and as prophylactic therapy in 2011.
Our study was retrospective in design; therefore, we could not avoid selection bias. However, our study had a relatively large sample size and no differences in demographic factors were observed between the two groups, which minimized the limitations. And, we have studied constant policies, including the method of surfactant instillation and ventilator care in the delivery room or neonatal intensive care unit for 6 years duration.
In conclusion, this is the first study to compare the efficacy of Curosurf® and Newfactan® as early prophylactic surfactant therapy in Korean neonates. We observed no differences in the clinical efficacy of Curosurf® and Newfactan® as early prophylactic surfactant therapy for RDS.
Figures and Tables
Fig. 1
Sequential changes in ventilator parameters over the 96 h after surfactant instillation. (A) The FiO2 (%) time course was not different between the Curosurf® and Newfactan® groups (P=0.784), (B) the mean airway pressure (MAP) time course was not different between the Curosurf® and Newfactan® groups (P=0.392); (C) the time course of arterial-to-alveolar oxygen pressure ratio was not different between the Curosurf® and Newfactan® groups (P=0.621); (D) the time course of the ventilatory index was not different between in the Curosurf® and Newfactan® groups (P=0.924).

Table 1
Demographic Factors of Infants and Mothers
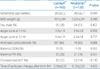
Table 2
Clinical Outcomes Associated with Respiratory Distress Syndrome

Table 3
Major Morbidities and Mortality Associated with Prematurity
References
2. Sandberg KL, Lindstrom DP, Sjöqvist BA, Parker RA, Cotton RB. Surfactant replacement therapy improves ventilation inhomogeneity in infants with respiratory distress syndrome. Pediatr Pulmonol. 1997; 24:337–343.

3. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2010 update. Zhonghua Er Ke Za Zhi. 2011; 49:27–33.
4. Engle WA. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008; 121:419–432.

5. Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014; 133:156–163.

7. Pramanik AK, Holtzman RB, Merritt TA. Surfactant replacement therapy for pulmonary diseases. Pediatr Clin North Am. 1993; 40:913–936.

8. Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001; (2):CD000510.

9. Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980; 1:55–59.

10. Choi CW, Hwang JH, Yoo EJ, Kim KA, Koh SY, Lee YK, et al. Comparison of clinical efficacy of Newfactan® versus Surfacten® for the treatment of respiratory distress syndrome in the newborn infants. J Korean Med Sci. 2005; 20:591–597.

11. Jeon GW, Oh M, Sin JB. Efficacy of surfactant-TA, calfactant and poractant alfa for preterm infants with respiratory distress syndrome: a retrospective study. Yonsei Med J. 2015; 56:433–439.

12. Kim SM, Park YJ, Chung SH, Choi YS, Kim CH, Bae CW. Early prophylactic versus late selective use of surfactant for respiratory distress syndrome in very preterm infants: a collaborative study of 53 multi-center trials in Korea. J Korean Med Sci. 2014; 29:1126–1131.

13. Kim JN, Shim EJ. Comparison of Curosurf® versus Surfacten® in the treatment of respiratory distress syndrome. Neonatal Med. 2013; 20:207–213.

14. Bae CW, Hahn WH, Chang JY, Kim SM. Surfactant replacement therapy for RDS: a collaborative study of 72 multi-center trials in Korea (2010) and a review of Korean experiences over 20 years. J Korean Soc Neonatol. 2011; 18:409–411.

15. Chung KY, Lee NM, Yun SW, Chae SA, Lim IS, Choi ES, et al. Comparison of outcomes between prophylactic and rescue therapy of surfactant in premature infants. Neonatal Med. 2013; 20:90–96.

16. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–1729.

17. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33:179–201.

18. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–534.

19. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16:128–140.

20. Choi BM, Lee KH, Eun BL, Yoo KH, Hong YS, Son CS, et al. Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics. 2005; 115:e255–e261.

21. Egberts J, de Winter JP, Sedin G, de Kleine MJ, Broberger U, van Bel F, et al. Comparison of prophylaxis and rescue treatment with Curosurf® in neonates less than 30 weeks' gestation: a randomized trial. Pediatrics. 1993; 92:768–774.

22. Ramanathan R. Animal-derived surfactants: where are we? the evidence from randomized, controlled clinical trials. J Perinatol. 2009; 29:Suppl 2. S38–S43.

23. Fujii AM, Patel SM, Allen R, Doros G, Guo CY, Testa S. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. J Perinatol. 2010; 30:665–670.

24. Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013; 163:955–960.

25. Hong SW, Lee EH, Kim SY, Park HJ. Comparison of the therapeutic effects of Curosurf® and Newfactan® in respiratory distress syndrome. J Korean Soc Neonatol. 2008; 15:142–150.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download