Abstract
Backgrounds/Aims
Mirizzi's syndrome (MS) poses great diagnostic and management challenge to the treating physician. We presented our experience of MS cases with respect to clinical presentation, diagnostic difficulties, surgical procedures and outcome.
Results
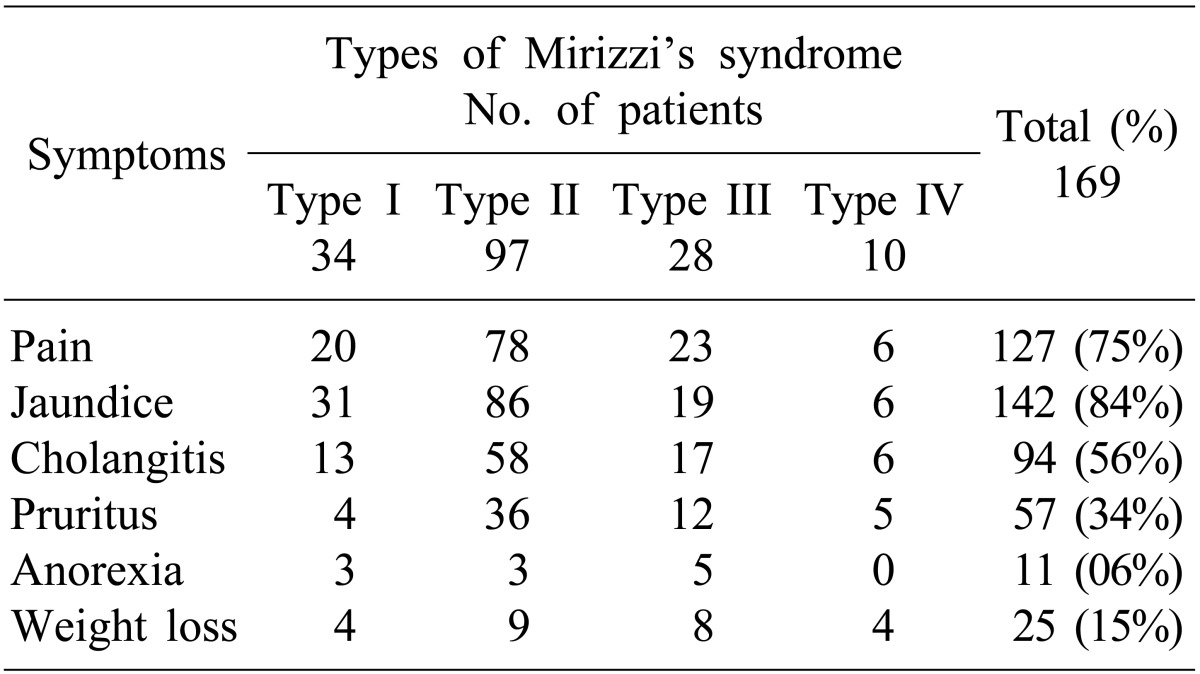
A total of 169 MS patients were surgically managed between 1989 and 2011. Presenting symptoms were jaundice (84%), pain (75%) and cholangitis (56%). Median symptom duration s was 8 months (range, <1 to 240 months). Preoperative diagnosis was possible only in 32% (54/169) of patients based on imaging study. Csendes Type II was the most common diagnosis (57%). Fistulization to the surrounding organs (bilio-enteric fistulization) were found in 14% of patients (24/169) during surgery. Gall bladder histopathology revealed xanthogranulomatous cholecystitis in 33% of patients (55/169). No significant difference in perioperative morbidity was found between choledochoplasty (use of gallbladder patch) (15/89, 17%) and bilio-enteric anastomosis (4/28, 14%) (p=0.748). Bile leak was more common with choledochoplasty (5/89, 5.6%) than bilio-enteric anastomosis (1/28, 3.5%), without statistical significance (p=0.669).
Conclusions
Preoperative diagnosis of MS was possible in only one-third of patients in our series. Significant number of patients had associated fistulae to the surrounding organs, making the surgical procedure more complicated. Awareness of this entity is important for intraoperative diagnosis and consequently, for optimal surgical strategy and good outcome.
Mirizzi's syndrome (MS) is a complication of gallstone disease, originally described by Pablo Louis Mirizzi as obstructive jaundice caused by a stone impacted at the neck of the gallbladder or at the cystic duct.1 Csendes et al.2 further added cholecysto-choledochal fistula and defined 4 evolving stages, from extrinsic compression to fistulization and complete erosion of the common hepatic duct. The clinical presentation of patients with MS varies from no symptoms to severe cholangitis. Preoperative diagnosis on the basis of clinical presentation and investigations is not possible in majority of patients.
Apart from masquerading as malignancy, MS is associated with increased risk of gallbladder cancer, as compared to patients with uncomplicated cholelithiasis.34 Bilio-enteric fistulae are also more common in these patients, affecting surgical management.5 Due to inflammation and complete obliteration of Calot's triangle, cholecystectomy, either by open or laparoscopic approach, poses a significant risk of bile duct injury. Among the numerous operative options described, subtotal cholecystectomy, choledochoplasty and bilio-enteric anastomosis are the commonly performed procedures,6 depending on the type. The optimal procedure is still not established between choledochoplasty and bilio-enteric anastomosis in Csendes Type II and III MS.6
In the present study, we analyzed clinical presentation, diagnostic difficulties, associated findings, surgical management and outcome of the MS cases we experienced.
A retrospective analysis was conducted on prospectively maintained database of all surgically treated patients with a final diagnosis of MS between 1989 and 2011 at a tertiary level referral and teaching hospital in north India. Demography, clinical presentation, preoperative imaging and intervention, operative findings were analyzed in detail. The final diagnosis of MS was based on intra-operative findings and patients were classified as per Csendes classification.2 Patient with associated malignancy were excluded from analyses of operative procedures and their outcome. In patients with cholecysto-choledochal fistula, suture repair of the common bile duct (CBD) defect (using gallbladder flap with or without 'T-tube') was considered as choledochoplasty. Follow-up data was collected from hospital records.
To evaluate the optimal procedure for type II & III MS, patients were categorized into 2 groups. 'Group 1' comprised patients who underwent choledochoplasty and 'Group 2' of those with bilio-enteric anastomosis. Post-operative morbidity was compared between the 2 groups.
Data analysis was performed using SPSS for Windows (SPSS Inc., Chicago, IL, USA), version 15.0. Chi-Square test was used for categorical variables. A p-value of <0.05 was considered significant.
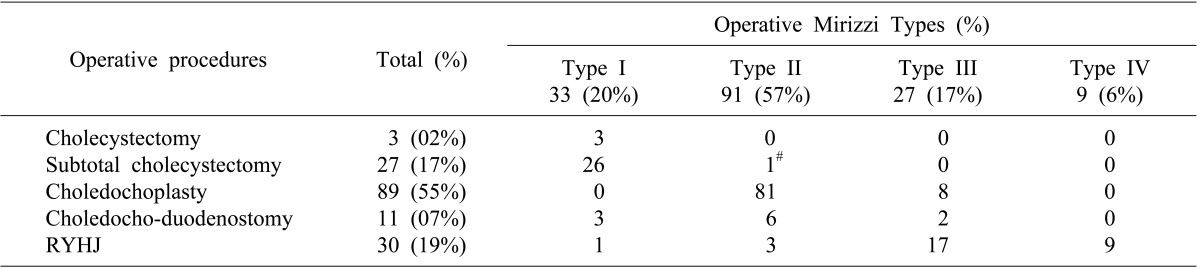
Between 1989 and 2011, 169 patients with MS were treated surgically in the department. The incidence of MS was 2.1% of all cholecystectomies (n≈8,000) performed during the same period. There were 62 (37%) males and 107 (63%) females with a median age of 53 years (range, 15 to 86). Overall median duration of symptoms was 8 months (range, <1 to 240 months). Clinical presentation was shown in Table 1. Type I MS was found in 34 (20%) patients, Type II in 97 (57%) patients, Type III in 28 (17%) patients and type IV in 10 (6%) patients.
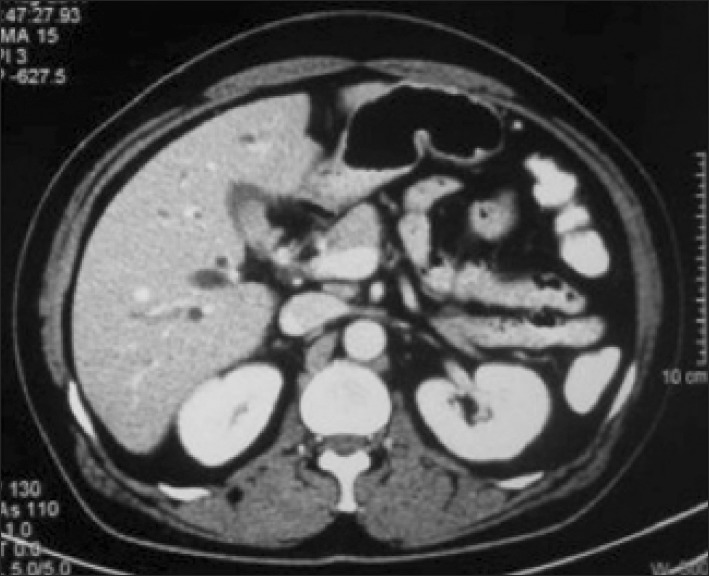
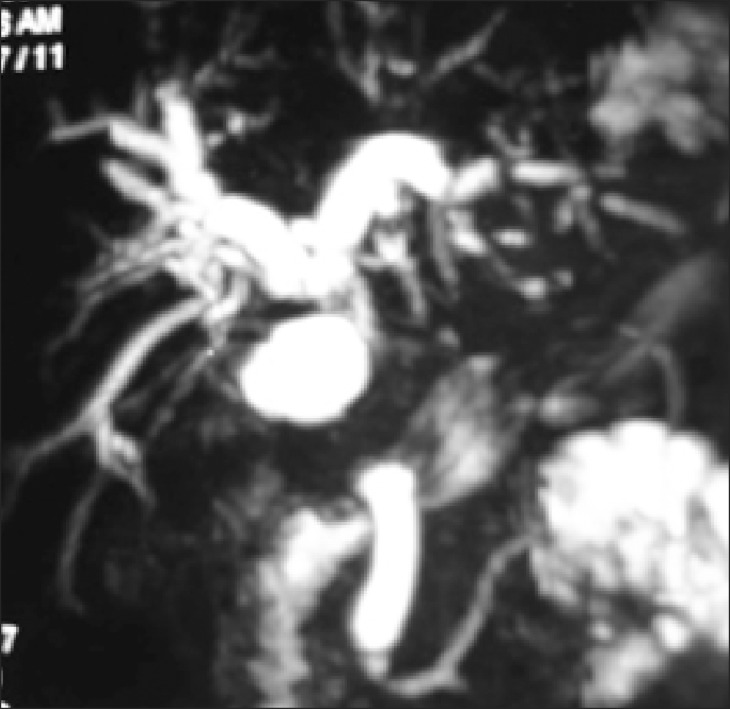
Trans-abdominal ultrasonography (USG) was conducted in all patients; however, it was diagnostic of MS only in 17 (10%) patients. Contrast-enhanced computed tomography (CT) scan was performed in 48 (28%) patients and only 5 were suspected as MS (Fig. 1). Endoscopic retrograde cholangiography (ERC) was conducted in 101 patients. The various indications of ERC were cholangitis (n=66, 65%), associated CBD stones (n=17, 17%) and diagnostic (n=18, 18 %). In addition, 42 out of the 101 patients underwent endoscopic stenting for cholangitis and/or failed clearance of CBD stones. Overall, the diagnosis of MS by ERC was possible in 27 (27%) patients. Since 2004, magnetic resonance cholangiography (MRC) has replaced diagnostic ERC at our center. Twelve patients with suspected MS underwent MRC; and MS was confirmed in 5 patients (Fig. 2). Preoperative fine needle aspiration cytology was conducted in 15 (7%) patients when the clinical suspicion of gall bladder cancer or cholangiocarcinoma was high. However, it was positive in only 1 patient. Overall, preoperative diagnosis of MS was possible in 54 (32%) patients.
Open surgical procedure was performed in 146 (86%) patients. The remaining 23 (14%) were attempted laparoscopically, with success in only 1 patient with Type II MS. Conversion from laparoscopic to open operation was necessitated by adhesions, unclear anatomy and frozen Calot's triangle.
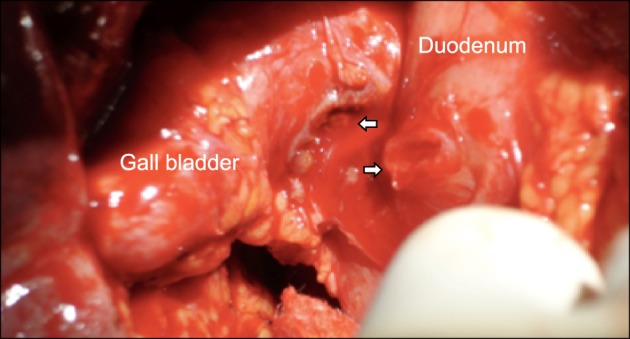
Common bile duct stones were present in 69 (41%) patients. Fistulization of the gallbladder to adjacent hollow viscera was encountered in 24 (14%) patients. Among these, fistulization to the duodenum was present in 18 (Fig. 3), colon in 4 and 1 patient each had fistulae to 2 organs (duodenum and colon in one, and stomach and duodenum in another). Other associated findings included significant pericholecystic adhesions in 111 (66%), gallbladder wall thickening in 142 (84%) and gallbladder mass in 2 patients. Six patients with suspected malignancy underwent intra-operative frozen section biopsy or fine needle aspiration cytology of gallbladder, 1 of which was positive for malignancy. The final histopathology was chronic cholecystitis (n=90, 53%), acute-on-chronic cholecystitis (n=16, 10%), xanthogranulomatous cholecystitis (n=55, 33%) and gallbladder cancer (n=8, 5%). One patient had carcinoma at head of pancreas with MS and xanthogranulomatous cholecystitis. Chronic cholecystitis with associated dysplasia was present in 4 patients.
Operative procedures performed included total or subtotal cholecystectomy, choledochoplasty and bilio-enteric anastomosis in the form of choledocho-duodenostomy or Roux-en-Y hepatico-jejunostomy. Since 2000, hepatico-jejunostomy has been the preferred method of bilio-enteric anastomosis wherever indicated. The details of surgical procedures performed in 160 patients without malignancy were listed in Table 2.
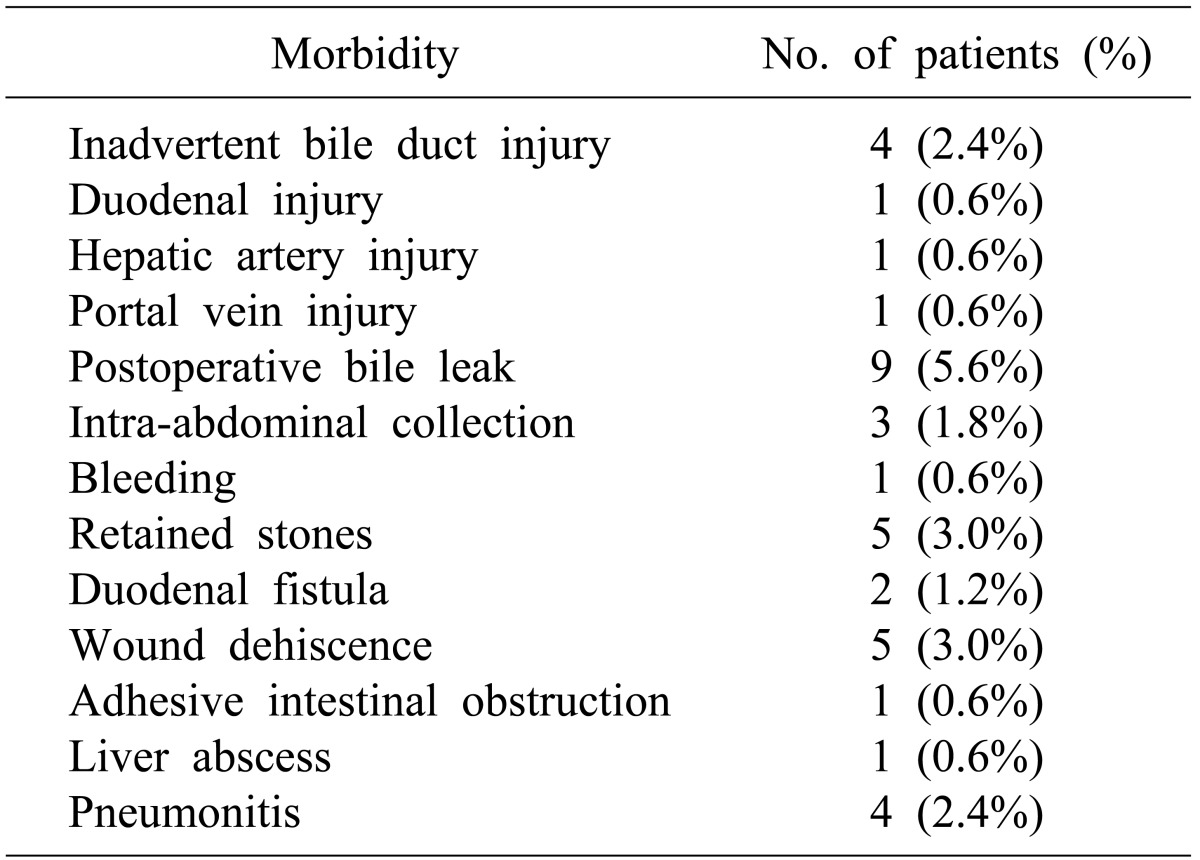
Twenty-eight patients (17.5%) had major perioperative morbidity (Table 3); and there was no peri-operative mortality. Patients with Mirizzi's type II and type III underwent either choledochoplasty or bilio-enteric anastomosis depending on operative findings and surgeon's preference. In subgroup analysis for choledochoplasty (Group1, n=89) and bilio-enteric anastomosis (Group 2, n=28) in patients with type II and III MS, perioperative morbidity occurred in 15 (17%) and 4 (14%) patients, respectively. Overall perioperative morbidity showed no significant differences (p=0.748). There was increased bile leak with choledochoplasty (5/89, 5.6%), as compared to bilio-enteric anastomosis (1/28, 3.5%), without statistical significance (p=0.669).
Follow-up details were available in 110 out of 160 (69%) patients, with a mean follow-up of 11 months (range 1-98 months). Two patients developed bile duct stricture following choledochoplasty for Type II MS. One of the patients subsequently needed Roux-en-Y hepaticojejunostomy. Other problems encountered were residual CBD stones in 4, mildly deranged liver function tests in 5 and portal hypertension with esophageal variceal bleed in 1 patient. One patient with benign hilar stricture (histopathologically proven), secondary biliary cirrhosis and Type II MS underwent Roux-en-Y hepaticojejunostomy. The patient was lost to follow-up due to death as a result of secondary biliary cirrhosis at 40 months post-surgery.
Pablo Luis Mirizzi, an Argentinean surgeon, described this complication of gallstone disease in 1948.1 Although he initially suspected it as a functional narrowing of the bile duct secondary to stone in the cystic duct, it was later recognized to be due to mechanical compression of the bile duct. Cholelithiasis with cholecysto-choledochal fistula was considered as an extension of the spectrum of MS and classified as Type II by Milone et al.7 Csendes et al.,2 in their largest reported series of 219 patients, further classified MS into 4 types and described surgical management options according to individual types. Type I was described as external compression by a stone in the cystic duct or Hartmann's pouch without cholecysto-choledochal fistula. Type II, III and IV were described as cholecysto-choledochal fistula, involving <one-third, one-third to two-third, and complete erosion of bile duct circumference, respectively. We have previously reported our experience of 14 patients with MS.8
Incidence of MS varies from 0.05-2.7% in patients with cholelithiasis9 and 0.7-1.4% of patients undergoing cholecystectomy.10 The relatively higher incidence of 2.1% in the present series could be explained by the high prevalence of gall stone disease in north India, late presentation (median duration of symptoms, 8 months) of patients for surgery and a referral bias.
Preoperative diagnosis of MS poses a clinical challenge, as the clinical presentation is nonspecific and usually similar to that of choledocholithiasis, as in our patients. Absence of jaundice as presenting symptom (14%) could be explained by patients with fistulizing variety of MS. It is occasionally possible to suspect or diagnose MS based on imaging. Ultrasonography and CT scan were diagnostic in only 10% of our patients. ERC is relatively more sensitive and offers a therapeutic opportunity in patients with cholangitis and CBD stones. However, ERC is invasive, hence MRC could be a better alternative with comparable results, as shown in our study. More frequent use of MRC in the preoperative period could increase the preoperative diagnosis of MS. Greiasov et al.11 reported a pre-operative diagnosis rate of 27% in a large cohort of 284 patients, similar to our series (32%). Recently, a small study of 16 patients indicated that intra-ductal ultrasound led to accurate diagnosis of all 9 patients with MS.12
Among the 4 types of MS, Type I is the most common in majority of case series.111314 In our experience, Type II MS was the most common, which corroborated other reports from Kashmir, India by Shah et al.15 and a large series from Chile by Csendes et al.2 This could be explained by the long standing disease in these populations with high gallstone prevalence.
Laparoscopic approach is currently used at many centers in select groups of MS patients. Chowbey et al.16 reported a 22% conversion rate in 27 MS patients by laparoscopic approach. Reviews of laparoscopic treatment of MS report a cumulative conversion rate up to 36.4%17 and 16% overall complication rate with bile duct injury as the most common complication.18 We prefer the open approach, as laparoscopic approach is associated with increased incidence of bile duct injury.
Radaelli et al.3 reported association of MS with gallbladder cancer in 28% of cases. They also found significantly higher levels of CA19-9 in these patients. In our patients, incidence of associated gallbladder cancer was lower (5%) and most were detected incidentally. Some authors advise routine frozen section biopsy in patients with MS.319 We conducted intraoperative frozen section biopsy or cytology selectively in 6 patients, but only 1 was positive. Xanthogranulomatous cholecystitis can mimic malignancy and is a surrogate marker of long-standing gallstone disease. It was observed in a relatively large number (n=55, 33%) of patients in our experience. Furthermore, the increased number (14%) of bilio-enteric fistulae could be explained by long duration of symptoms and a higher incidence of associated xanthogranulomatous cholecystitis, which has the tendency to erode into the adjacent organs.
Surgical management of Csendes type I and IV MS is well established, with subtotal cholecystectomy as the procedure of choice in Type I MS and cholecystectomy with Roux-en-Y hepatico-jejunostomy for Type IV MS. However, for small to moderate size cholecysto-choledochal fistula, i.e. Csendes type II and type III MS, the procedure of choice for type II and type III MS is unclear. Both procedures (choledochoplasty and bilio-enteric anastomosis) are previously reported, similar to our series. Some authors have raised concerns of choledochoplasty, as direct repair using gallbladder flap is prone to failure due to associated inflammatory process; rather, they recommend bilio-enteric anastomosis.2021 In our series, no significant difference was observed in overall morbidity on comparing choledochoplasty (89/169, 55%) with bilio-enteric anastomosis (41/169, 26%) in type II and type III MS. However, the incidence of bile leak was 5.6% and 3.5% in choledochoplasty and bilio-enteric anastomosis group, respectively, without statistical significance. In our study, 2 patients developed stricture at follow-up after choledochoplasty for type II MS; of which, 1 patient subsequently underwent Roux-en-Y hepaticojejunostomy. Based on this observation, we are more inclined towards bilio-enteric anastomosis for type II and III MS.
Major morbidity in our series was 17.5%, which is higher than other reported series.215 This could be due to a higher number of fistulas to adjacent organs and associated xanthogranulomatous cholecystitis. Involvement of multiple trainee surgeons may be another contributing factor.
In conclusion, preoperative diagnosis of MS based on clinical presentation and imaging findings was possible in only one third of patients in our series. Increased association of fistulization to surrounding organs, adhesions, CBD stones, bilio-enteric fistulae and gall bladder cancer influenced surgical approach and operative difficulty. Subtotal cholecystectomy and Roux-en-Y hepaticojejunostomy were procedures of choice in Type I and IV MS, respectively. For patients with Type II and III MS, choledochoplasty was more often associated with postoperative bile leak than bilio-enteric anastomosis; and we now tend to favor bilio-enteric anastomosis. Awareness of this entity is important for intraoperative diagnosis, and consequently, for optimal surgical strategy and good outcome.
References
2. Csendes A, Díaz JC, Burdiles P, Maluenda F, Nava O. Mirizzi syndrome and cholecystobiliary fistula: a unifying classification. Br J Surg. 1989; 76:1139–1143. PMID: 2597969.

3. Redaelli CA, Büchler MW, Schilling MK, Krähenbühl L, Ruchti C, Blumgart LH, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997; 121:58–63. PMID: 9001552.

4. Prasad TL, Kumar A, Sikora SS, Saxena R, Kapoor VK. Mirizzi syndrome and gallbladder cancer. J Hepatobiliary Pancreat Surg. 2006; 13:323–326. PMID: 16858544.

5. Beltran MA, Csendes A, Cruces KS. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg. 2008; 32:2237–2243. PMID: 18587614.

6. Tan KY, Chng HC, Chen CY, Tan SM, Poh BK, Hoe MN. Mirizzi syndrome: noteworthy aspects of a retrospective study in one centre. ANZ J Surg. 2004; 74:833–837. PMID: 15456425.

7. Milone M, Musella M, Maietta P, Guadioso D, Pisapia A, Coretti G, et al. Acute acalculous cholecystitis determining Mirizzi syndrome: case report and literature review. BMC Surg. 2014; 14:90. PMID: 25399060.

8. Ibrarullah M, Saxena R, Sikora SS, Kapoor VK, Saraswat VA, Kaushik SP. Mirizzi's syndrome: identification and management strategy. Aust N Z J Surg. 1993; 63:802–806. PMID: 8274124.

9. Johnson LW, Sehon JK, Lee WC, Zibari GB, McDonald JC. Mirizzi's syndrome: experience from a multi-institutional review. Am Surg. 2001; 67:11–14. PMID: 11206888.
10. Abou-Saif A, Al-Kawas FH. Complications of gallstone disease: Mirizzi syndrome, cholecystocholedochal fistula, and gallstone ileus. Am J Gastroenterol. 2002; 97:249–254. PMID: 11866258.

11. Greiasov VI, Perfil'ev VV, Shchepkin SP, Petrichenko AV, Sivokon NI, Chuguevskiĭ VM. Diagnostics and surgical tactics for Mirizzi syndrome. Khirurgiia (Mosk). 2008; (11):31–34. PMID: 19301493.
12. Moon JH, Cho YD, Cheon YK, Ryu CB, Kim YS, Kim JO, et al. Wire-guided intraductal US in the assessment of bile duct strictures with Mirizzi syndrome-like features at ERCP. Gastrointest Endosc. 2002; 56:873–879. PMID: 12447301.

13. Al-Akeely MH, Alam MK, Bismar HA, Khalid K, Al-Teimi I, Al-Dossary NF. Mirizzi syndrome: ten years experience from a teaching hospital in Riyadh. World J Surg. 2005; 29:1687–1692. PMID: 16311870.

14. Safioleas M, Stamatakos M, Safioleas P, Smyrnis A, Revenas C, Safioleas C. Mirizzi syndrome: an unexpected problem of cholelithiasis. Our experience with 27 cases. Int Semin Surg Oncol. 2008; 5:12. PMID: 18495037.

15. Shah OJ, Dar MA, Wani MA, Wani NA. Management of Mirizzi syndrome: a new surgical approach. ANZ J Surg. 2001; 71:423–427. PMID: 11450919.

16. Chowbey PK, Sharma A, Mann V, Khullar R, Baijal M, Vashistha A. The management of Mirizzi syndrome in the laparoscopic era. Surg Laparosc Endosc Percutan Tech. 2000; 10:11–14. PMID: 10872519.

17. Yeh CN, Jan YY, Chen MF. Laparoscopic treatment for Mirizzi syndrome. Surg Endosc. 2003; 17:1573–1578. PMID: 12964062.

18. Antoniou SA, Antoniou GA, Makridis C. Laparoscopic treatment of Mirizzi syndrome: a systematic review. Surg Endosc. 2010; 24:33–39. PMID: 19466486.

19. Schäfer M, Schneiter R, Krähenbühl L. Incidence and management of Mirizzi syndrome during laparoscopic cholecystectomy. Surg Endosc. 2003; 17:1186–1190. PMID: 12739118.
20. Baer HU, Matthews JB, Schweizer WP, Gertsch P, Blumgart LH. Management of the Mirizzi syndrome and the surgical implications of cholecystcholedochal fistula. Br J Surg. 1990; 77:743–745. PMID: 2383747.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download