Abstract
Very rarely, spinal subarachnoid hemorrhage (SSAH) can occur without any direct spinal injury in patients with traumatic intracranial SAH. A-59-year-old male with traumatic intracranial subarachnoid hemorrhage (SAH) presented with pain and numbness in his buttock and thigh two days after trauma. Pain and numbness rapidly worsened and perianal numbness and voiding difficulty began on the next day. Magnetic resonance imaging showed intraspinal hemorrhage in the lumbosacral region. The cauda equina was displaced and compressed. Emergent laminectomy and drainage of hemorrhage were performed and SSAH was found intraoperatively. The symptoms were relieved immediately after the surgery. Patients with traumatic intracranial hemorrhage who present with delayed pain or neurological deficits should be evaluated for intraspinal hemorrhage promptly, even when the patients had no history of direct spinal injury and had no apparent symptoms related to the spinal injury in the initial period of trauma.
Traumatic spinal subarachnoid hemorrhage (SSAH) and spinal subdural hemorrhage (SSDH) are very rare. Most cases occur as a complication of spinal procedures such as spinal anesthesia and are often associated with blood coagulation abnormalities, such as anticoagulation therapy or thrombocytopenia.2) Traumatic SSAH or SSDH can occur at the site of the spinal injury, but several cases of traumatic SSAH or SSDH that was supposed to be migration of intracranial traumatic subarachnoid hemorrhage (SAH) or subdural hemorrhage (SDH) without direct spinal injuries were reported.12368) Of them, reports of traumatic SSAH migrated from intracranial traumatic SAH are extremely rare.2)
Here, we report a case of SSAH migrated from traumatic intracranial SAH in a patient without direct spinal injury.
A-59-year-old male presented with headache after traumatic brain injury (TBI). He had hit his head on the corner of a desk and had lost his consciousness a few seconds an hour before. He did not complain of pain in other parts of his body except for his headache. On neurological examination, Glasgow Coma Scale was 15 and no motor or sensory changes were found. He had had a history of TBI and had undergone bilateral craniotomy and removal of epidural hematoma (EDH) 5 years prior. He had no previous medical problems and took no medication. A brain computed tomography (CT) scan revealed EDH in the right frontal lobe and traumatic SAH in the ambient cistern (Figure 1). Due to his stable clinical condition, no immediate surgical treatment was planned. Instead, he was admitted for close monitoring of his neurological status.
Two days after TBI, he complained of pain and numbness in the bilateral buttock and posterior thigh. A lumbar spine plain radiograph showed no fracture or dislocation. His pain and numbness progressively worsened. On the next day, he could not sit or stand due to severe pain and numbness. Perianal numbness and voiding difficulty began. A Foley catheter was placed and 500 cc of urine was drained. On neurological examination, motor weakness in the lower extremities was not evident. A follow-up brain CT scan showed a slight decrease in the thickness of EDH, and SAH was much resolved (Figure 2). Lumbar spine magnetic resonance imaging (MRI) revealed a fusiform lesion within the dorsal part of the spinal canal at the L5 and S1 level. The cauda equina was compressed and displaced laterally. The lesion had low signal intensity in the T2-weighted image (WI) and iso-signal intensity in the T1-WI, suggestive of acute hemorrhage (Figure 3). Contrast enhancement images showed no abnormally enhanced lesion.
A diagnosis of cauda equina syndrome due to intraspinal hemorrhage was made and emergent operation was performed. After a partial laminectomy of S1, the dural sac was exposed and no EDH was found. The dural sac was tense on palpation. When opening the dura, SDH was not found. On incision of the arachnoid membrane, dark reddish fluid gushed out with high pressure. No blood clot and no definite bleeding point were found. The pain and numbness disappeared immediately after the surgery. He had gradual improvement in his voiding function. At his 1-year-follow-up, he had no subjective discomfort in his voiding function.
Traumatic intracranial hemorrhage can cause intraspinal hemorrhage in patients without any direct injury to the spine as in the present case.12368) SSDH can occur in patients with traumatic intracranial SDH.168) Because of the anatomic continuity between the intracranial and spinal subdural spaces, redistribution or migration of the intracranial SDH to the spinal subdural space can occur.16) If an arachnoid tear exists, this might facilitate spontaneous resolution and migration of intracranial SDH by a dilutional and "water hammer" like effect of cerebrospinal fluid (CSF) that leaks from the subarachnoid space to the subdural space through the arachnoid tear.8) Furthermore, raised intracranial pressure due to intracranial SDH can push the hematoma directly into the skull base and spinal canal through the subdural space.4)
Although not a traumatic etiology, cases of SSDH occurring in patients with intracranial SAH have been reported.47) SAH under high pressure can tear the intact arachnoid membrane, extravasate the SAH into the subdural space, and cause intracranial SDH or SSDH.4) If an arachnoid tear already exists, intracranial SAH might migrate into the subdural space through the arachnoid tear, and this can develop into intracranial SDH or SSDH.4)
Cerebral contusion can also cause SSAH. There was a case report of SSAH in a patient with cerebral contusion in the temporal lobe.3) One day after admission, the patient complained of neck pain, followed by severe right hemiparesis, dysphagia, and hoarseness. CT scan revealed progression of the contusion and intraspinal hemorrhage extending to the C3 level with the brain stem and the spinal cord displaced contralaterally. SSAH was detected intraoperatively and the authors suggested that the progression of the initial temporal lobe contusion made the blood spread into the adjacent subarachnoid spaces. This intracranial SAH might have migrated to the upper cervical level. The cisterns of the posterior cranial fossa include the prepontine, interpeduncular, cerebellopontine, premedullary, cerebellomedullary, quadrigeminal, superior cerebellar cisterns, and the cisterna magna. The ambient cistern communicates with the interpeduncular cistern. The anterior and posterior spinal cisterns communicate with cisterns of the posterior cranial fossa through the foramen magnum. Therefore, intracranial SAH can migrate to the spinal subarachnoid space.3) This migration occurs with higher probability in patients with a large amount of SAH or after early ambulation of the patient.3)
SSAH can occur in patient with traumatic intracranial SAH as in the present case. Chen et al.2) reported a case of SSAH in a patient with traumatic SAH within the left sylvian cistern. After admission, the patient began complaining of numbness in the left posterior thigh and a heavy sensation in the legs. Lumbar spine MRI 9 days after the initial trauma showed a fusiform lesion located at the L5 and S1 level. During surgery, an old blood clot was removed after opening the arachnoid membrane. The authors hypothesized that migration of blood could have occurred from the intracranial subarachnoid space to the subarachnoid space at the most caudal part of the spinal canal.2) In the present case, the patient complained of no low back or buttock pain immediately after the trauma. The patient presented pain and numbness with delayed onset and symptoms worsened rapidly. Intracranial SAH was much resolved and no traumatic lesion in the lumbosacral spine was found in the radiologic evaluation when the patient began complaining of pain. Therefore, we hypothesize that the SSAH might have been related to progressive migration of intracranial SAH in the ambient cistern to the most dependent area of the lumbosacral subarachnoid space through the above mentioned pathways. Contrary to Chen et al.'s2) report, we found uncoagulated dark red blood rather than an old blood clot intraoperatively. In our opinion, this might have resulted from the different timing of the operation. In the present case, operation was performed 3 days after the initial trauma, as compared to surgery being performed 9 or more days after the trauma in Chen et al.'s2) report. SSAH rarely forms a hematoma owing to the continuous diluting or "washing" effect of the CSF.25) Interestingly, migration of the blood can occur in reverse direction, that is, from the spine to the brain.910) Although not a traumatic etiology, there were reports in which intracranial SAH or SDH was found later during the workup for SSDH or SSAH.910) In the present case, although the onset of symptoms was delayed, the deterioration of symptoms was rapid, as in other reports.138) Therefore, a suspicion of intraspinal hemorrhage and prompt radiologic workup should be performed in patients with traumatic intracranial SAH or SDH who present with delayed pain or neurologic deficit.
We suggest that delayed intraspinal hemorrhage may occur in patients with TBI. In particular, patients who have traumatic intracranial SAH or SDH and who present with delayed pain or neurological deficits should be evaluated for SSAH or SSDH promptly, even when the patients had no history of direct spinal injury and had no apparent symptoms related to the spinal injury in the initial period of trauma.
References
1. Bortolotti C, Wang H, Fraser K, Lanzino G. Subacute spinal subdural hematoma after spontaneous resolution of cranial subdural hematoma: causal relationship or coincidence? Case report. J Neurosurg. 2004; 100:372–374. PMID: 15070147.
2. Chen HC, Hsu PW, Tzaan WC. "Migration" of traumatic subarachnoid hematoma? A case report. Surg Neurol. 2008; 70:213–216. PMID: 17720228.

3. Di Rienzo A, Iacoangeli M, Alvaro L, Colasanti R, Moriconi E, Gladi M, et al. Subarachnoid hematoma of the craniocervical junction and upper cervical spine after traumatic cerebral contusion: case report. Neurol Med Chir (Tokyo). 2013; 53:620–624. PMID: 24067775.

4. Jung HS, Jeon I, Kim SW. Spontaneous spinal subdural hematoma with simultaneous cranial subarachnoid hemorrhage. J Korean Neurosurg Soc. 2015; 57:371–375. PMID: 26113966.

5. Kakitsubata Y, Theodorou SJ, Theodorou DJ, Miyata Y, Ito Y, Yuki Y, et al. Spontaneous spinal subarachnoid hemorrhage associated with subdural hematoma at different spinal levels. Emerg Radiol. 2010; 17:69–72. PMID: 19184145.

6. Kim HG, Kim TW, Park KH, Chi MP. Traumatic spinal subdural hematoma with intracranial subdural hematoma. Korean J Neurotrauma. 2014; 10:146–148. PMID: 27169053.

7. Mete A, Erkutlu I, Akcali A, Mete A. Simultaneous cranial subarachnoid hemorrhage and spinal subdural hematoma. Turk Neurosurg. 2012; 22:349–352. PMID: 22665005.

8. Moscovici S, Paldor I, Ramirez de-Noriega F, Itshayek E, Shoshan Y, Spektor S, et al. Do cranial subdural hematomas migrate to the lumbar spine? J Clin Neurosci. 2011; 18:563–565. PMID: 21257311.

9. Peñas ML, Guerrero AL, Rodríguez Velasco M, Herrero S. Spontaneous intradural spinal haematoma associated with a cerebral subarachnoid haemorrhage. Neurologia. 2011; 26:182–184. PMID: 21163233.

10. Sasaji T, Shinagawa K, Matsuya S. Spontaneous thoracic spinal subarachnoid hemorrhage diagnosed with brain computed tomography. Tohoku J Exp Med. 2013; 231:139–144. PMID: 24131866.

FIGURE 1
(A) Brain computed tomography scan shows epidural hematoma in the right frontal lobe and (B) subarachnoid hemorrhage in the ambient cistern (arrow).
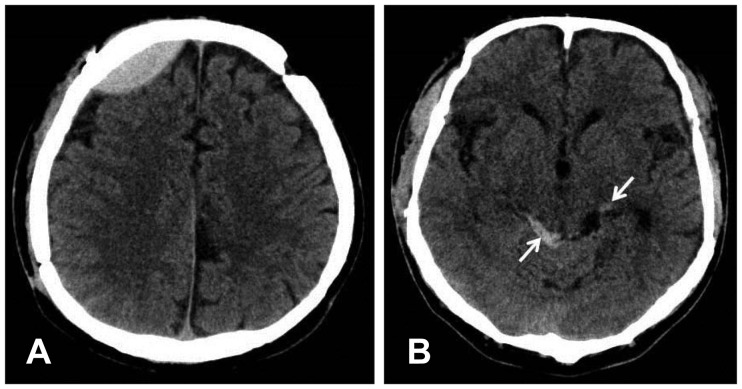
FIGURE 2
(A) Brain computed tomography scan shows decrease in the thickness of epidural hematoma and (B) resolution of subarachnoid hemorrhage (arrow).
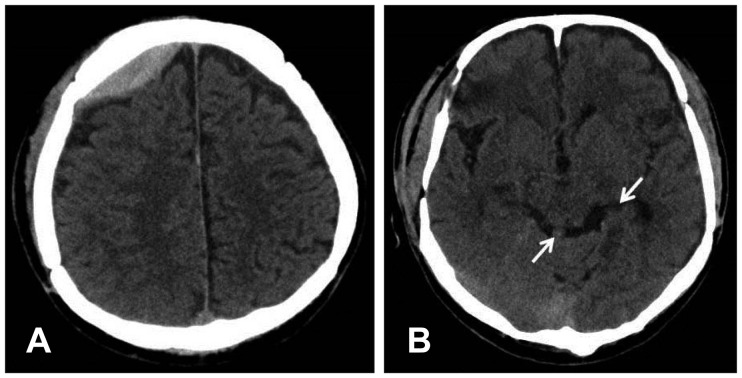
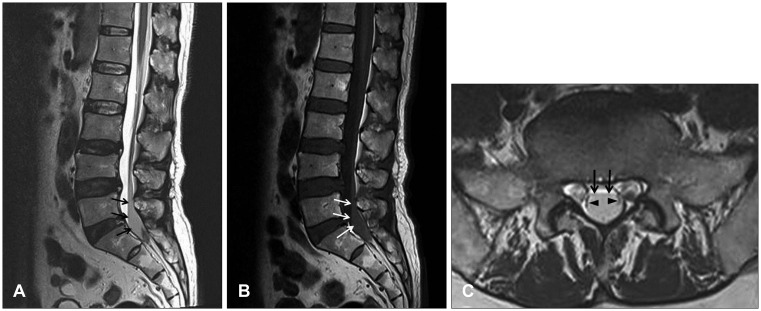
FIGURE 3
(A) Lumbar spine magnetic resonance imaging shows fusiform lesions with low signal intensity in the T2-sagittal image (arrow) and (B) iso-signal intensity in the T1-sagittal image (arrow) (C) within the dorsal aspect of the spinal canal at the L5 and S1 level. T2-axial image shows low signal intensity lesion within the thecal sac (arrow) which displaces the cauda equina laterally (arrow head).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download