Abstract
Background
N-containing bisphosphonates (BPs), such as pamidronate and risedronate, can inhibit osteoclastic function and reduce osteoclast number by inducing apoptotic cell death in osteoclasts. The aim of this study is to demonstrate the effect of pamidronate, second generation nitrogen-containing BPs and to elucidate matrix metallo-proteinases (MMPs) mRNA expression under serum starvation and/or tumor necrosis factor alpha (TNF-α) stimulation on metabolism of intervertebral disc (IVD) cells in vitro.
Methods
Firstly, to test the effect of pamidronate on IVD cells in vitro, various concentrations (10-12, 10-10, 10-8, and 10-6 M) of pamidronate were administered to IVD cells. Then DNA and proteoglycan synthesis were measured and messenger RNA (mRNA) expressions of type I collagen, type II collagen, and aggrecan were analyzed. Secondly, to elucidate the expression of MMPs mRNA in human IVD cells under the lower serum status, IVD cells were cultivated in full serum or 1% serum. Thirdly, to elucidate the expression of MMPs mRNA in IVD cells under the stimulation of 1% serum and TNF-α (10 ng/mL) In this study, IVD cells were cultivated in three dimensional alginate bead.
Results
Under the lower serum culture, IVD cells in alginate beads showed upregulation of MMP 2, 3, 9, 13 mRNA. The cells in lower serum and TNF-α also demonstrated upregulation of MMP-2, 3, 9, and 13 mRNA. The cells with various doses of pamidronate and lower serum and TNF-α were reveled partial down-regulation of MMPs.
Nitrogen-containing bisphosphonates (BPs), such as pamidronate, alendronate, and risedronate, can inhibit osteoclastic function and reduce osteoclast number by inducing apoptotic cell death in osteoclasts.[1234] Long-term BP administration significantly reduces bone and calcified cartilage resorption through impairment of cellular structure and bone-resorbing function of osteoclasts via inhibiting osteoclastic adhesion to bone matrix, and thereby effectively maintains trabecular bone volume and structure.[5] Several molecular targets for nitrogen-containing BPs have been suggested. BPs inhibit enzymes of the mevalonate pathway and prevent the biosynthesis of isoprenoid compounds, which are essential for the post-translational modification of small guanosine triphosphatases (GTPases), which is crucial for osteoclastic adhesion and ruffle border formation.[34] BPs thus decrease osteoclast function and induce their apoptosis.[23]
As potent inhibitors of bone resorption, they are widely used for the treatment of bone disorders that are due to increased osteoclast activity i.e., postmenopausal osteoporosis, Paget's disease, tumoral bone disease, and particle induced osteolysis around metal implant.[678] Recent evidences demonstrate BPs in therapeutic concentrations are safe for articular chondrocytes in vitro, moreover, pamidronate and risedronate prevent dexamethasone induced growth retardation and apoptosis of chondrocytes.[9] In addition to that, BPs also has been known to decrease breakdown of type II collagen of articular cartilage [10] and exhibit partial chondroprotective effect in a rabbit model of inflammatory arthritis.[11] These findings support the fact that BPs have a chondroprotective effect via maintenance of chondrogenic phenotypes, anti-apoptosis, and protection against cartilage degradation.
Intervertebral disc (IVD) degeneration is characterized in part by a progressive decrease in proteoglycan content leading to dehydration of the nucleus pulposus [12131415] causing degenerative changes of the spine.[16] Among mechanisms responsible for IVD degeneration, apoptosis [171819] and over-expression [19202122] of matrix breakdown enzymes is of importance since these mechanisms account final phenomenon of degeneration such as cell deaths and depletion of chondrogeneic IVD matrix. Degenerated IVD spontaneously secretes various cytokines and enzymes, which includes interleukin-1, -6, prostaglandins, nitric oxide, tumor necrosis factor alpha (TNF-α), and matrix metalloproteinases (MMPs).[2324] Furthermore, in the model of IVD degeneration, MMPs and adamalysin with thrombospondin motifs (ADAMTS) play crucial role in pathogenesis of IVD degeneration.[2526]
There have been several reports of the effect of BPs on chondrocytes and articular cartilage, which are mainly anti-apoptotic, proliferative and chondroprotective effects. [91011] In cartilage degradation, aggrecan was degraded by ADAMTS-5 and collagens were degraded by MMP-13.[27] However, BP (alendronate) down-regulates ADAMTS-4, -5 in chondrocyte and epiphyseal cartilage.[28] Furthermore BPs down-regulated the expression of MMP-1, -2, -3, -7, -8, -9, -12, -13, and -14 in various cell line indicate possibility of antiresorptive and anti-metastatic effect of BPs in degenerative and metastatic disease.[29]
The fact that BPs has a powerful chondroprotective effect and antiresorptive effect by inhibiting MMPs and ADAMTS prompts a research for the sum effect of BPs on IVDs in terms of various chondrogenic phenotype and MMPs expression. Nevertheless, the effect of BPs on IVDs in vitro was not thoroughly elucidated before. Especially, wide spread prescription and long term treatment of BPs for postmenopausal osteoporosis more than ten years renders an important question of how IVD react to BPs.[30] In addition to that, IVD degeneration might be benefited from long-term treatment of BPs in terms of IVD matrix protection via phenotypical stabilization and down-regulation of MMPs. Thus, we hypothesized that BPs, powerful chondroprotective and antiresorptive, can protect chondrogenic phenotype by inhibiting breakdown of aggrecan and type II collagen via a mechanism which is mediated MMPs-dependent matrix degradation.
Accordingly, the purposes of this experimental study were to demonstrate the effect of pamidronate, second generation nitrogen-containing BPs, on in vitro metabolism of IVD cells, cellular proliferation, matrix synthesis, phenotypical expression and to elucidate MMP-1, -2, -3, -9, and -13 messenger RNA (mRNA) expression under serum starvation and/or TNF-α stimulation in human IVD cells.
All of the experimental protocols were approved by the human subjects Institutional Review Board of Yonsei University.
Firstly, to test the effect of pamidronate on IVD cells in vitro, human IVD cells were utilized. Various concentrations (10-12, 10-9, 10-6, and 10-3 M) of pamidronate were administered to IVD cells and cultured in three dimensional alginate beads. Then DNA and proteoglycan synthesis were measured and mRNA expressions of type I collagen, type II collagen, and aggrecan were analyzed by reverse transcription-polymerase chain reaction (RT-PCR).
Secondly, to elucidate the expression of MMP-1, -2, -3, -9, and -13 mRNA in human IVD cells under the stimulation of lower serum status (1% fetal bovine serum [FBS] culture), human IVD cells were cultivated in three dimensional alginate bead in full serum or 1% serum. Pamidronate was administered to each culture with various dosages (10-12, 10-10, 10-8, and 10-6 M).
Thirdly, to elucidate the expression of MMP-1, -2, -3, -9, and -13 mRNA in human IVD cells under the stimulation of lower serum status (1% FBS culture) and TNF-α (10 ng/mL), human IVD cells were cultivated in three dimensional alginate bead in full serum or 1% serum with or without TNF-α (10 ng/mL). Pamidronate was administered to each culture with various dosages (10-12, 10-10, 10-8, and 10-6 M).
Lumbar IVD tissues were obtained from eight patients (age range, 38-48 years) during surgical disc procedures. Classification of the IVD of each patient as grade of degeneration was performed based on magnetic resonance images of each disc as described in the literature.[31] Grade III and IV degenerations were included in this study to minimize the effect of degeneration grades on the expression of phenotype and matrix synthesis. An attempt was made by the operating surgeon to carefully obtain tissue from the central aspect of the disc to optimize harvest of only the nucleus pulposus and transitional zone. Herniated disc material was strictly excluded from current study. The disc tissue specimens were washed with Hank's balanced salt solution (HBSS; Gibco-BRL, Grand Island, NY, USA) to remove blood and bodily fluid contaminants, and were then transported in sterile HBSS to the laboratory, less than 20 min following surgical removal.
Any obvious granulation tissue, dense outer annulus, and cartilaginous endplate were removed carefully from the disc tissue specimens. Disc cells were then isolated from the inner annulus and nucleus pulposus as described before.[32] Briefly the dissected specimens were minced with a scalpel into pieces of approximately two cubic millimeters in volume. Disc tissues from the nucleus pulposus and inner annulus were digested for 60 min at 37℃ under gentle agitation in a medium composed of equal parts of Dulbecco's modified Eagle's medium and Ham's F-12 medium (DMEM/F12; Gibco-BRL) containing 5% heat-inactivated FBS (Gibco-BRL) with 0.2% pronase (Sigma Chemical Co., St. Louis, MO, USA) and 0.004% deoxyribonuclease II type IV (DNase; Sigma Chemical Co.). The tissue was then washed 3 times with DMEM/F12 (Gibco-BRL) and digested overnight under the same conditions, except that the pronase was replaced with bacterial 0.02% collagenase type II (Worthington Biochemical Corp., Freehold, NJ, USA). Cells were filtered through a sterile nylon mesh filter (pore size=75 µm; BD Falcon, Franklin Lakes, NJ, USA) and then were counted in a hematocytometer and plated in 24 well plates (BD Falcon) at a density of approximately 5×105 cells/mL. Primary cultures were sustained for 2 to 3 weeks in DMEM/F12 containing 10% FBS, 1% v/v penicillin, streptomycin and nystatin (all antibiotics from Gibco-BRL) in a 5% CO2 incubator with humidity. Culture medium was changed twice a week. Cell viability was determined by trypan blue exclusion test.[32]
The preparation of IVD cells in alginate beads was performed as described elsewhere.[25] Briefly, isolated cells from primary culture with trysinization were resuspended in sterile 150 mM NaCl containing 1.2% low-viscosity alginate (Sigma Chemical Co.) at a density of one million cells per milliliter, and then slowly expressed through a 22 gauge needle in a drop-wise fashion into 102 mM CaCl2 solutions. After gelation, the beads were allowed to polymerize further for a period of 10 min in the CaCl2 solution. After wash in 10 times volume of 150 mM NaCl and three times washes in 10 times volume of DMEM/F12 (Gibco-BRL). The beads were finally placed in complete culture medium. Ten beads were cultured in each well of a 24-well plate (BD Falcon).
To remove cells from the alginate bead, wells were rinsed twice with 150 mM with gentle pipetting into the well. The rinse solution was incubated for 1 min and was aspirated off. Three times the volume of alginate in dissolving buffer (55 mM sodium citrate and 150 mM NaCl) was added to the wells and plates were incubated at 37℃ for 10 min with shaking.
Total cellular RNA was isolated using the TRIzol reagent (Gibco-BRL). The complementary DNA (cDNA) was synthesized from 3 µg total RNA using murine leukemia virus RT (MLV RT; Promega, Madison, WI, USA) with the oligo (dT) priming method in a 50 µL reaction mixture. 2 µL aliquots were amplified in a 20 µL reaction mixture that contained 1U Taq DNA polymerase (Bioneer KAIST, Daejeon, Korea), 250 µM of each deoxynucleotide (dNTP), 10 mM Tris-hydrochloride (HCl; pH9.0), 40 mM KCl and 1.5 mM MgCl2. The same reaction profile was used for all primer sets: an initial denaturation at 94℃ for 1 min, followed by 25 cycles of: 94℃ for 5 sec; 47 to 50℃ for 5 sec; and 72℃ for 30 sec; and an additional 2 min extension step at 72℃ after the last cycle. Amplification reactions specific for the following cDNAs were performed: Beta-actin, collagen type I alpha, collagen type II and aggrecan. Primer sequence of each cDNA was listed on Table 1. PCR products (5 µL) were analysed by electrophoresis in 2% agarose gels, and detected by staining with ethidium bromide. The intensity of the products was quantified using the BioImage Visage 110 system (BioRad, Hercules, CA, USA).
IVD cell proliferation after 48 hr was determined by a 3H-thymidine incorporation method.[33] All experiments were done in quadruplicate. Results were expressed as counts per min (cpm).
Cultures were administered with fresh Newman-Tytell serumless medium containing 35S-sulfate (final concentration 20 µCi/mL). After a period of 4 hr, cultures with labeling medium were extracted at 4℃ for 48 hr by addition of an equal volume of 8 M guanidine HCl, 20 mM ethylenediaminetetraacetic acid (EDTA) and a mixture of proteinase inhibitors (Sigma Chemical Co.). For quantitative evaluation of 35S-sulfate labeled proteoglycans, aliquots of the stored extracts were eluted on Sephadex G-25M column (PD-10; Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) under dissociative conditions. The radioactivities of the newly synthesized proteoglycans were measured by scintillation counting (Packard BioScience, Meriden, CT, USA). All data was normalized by 3H-thymidine incorporation, which was measured at the end of culture as described elsewhere.
One-way analysis of variance with Fisher's protected least significant difference (LSD) post-hoc test was performed to test difference in densitometric data and 3H-thymidine incorporation, 35S-sulfate labeled proteoglycan. All statistical analyses were performed using SPSS statistical software (version 18.0; SPSS Inc., Chicago, IL, USA). A probability value of P<0.05 was considered significant.
Human IVD cell culture in three dimensional alginate beads with various concentrations of pamidronate (10-12, 10-9, 10-6, and 10-3 M) showed no significant increase in DNA synthesis compared to control culture without pamidronate, indicating there was neither cellular proliferative nor cytotoxic effect of pamidronate on human intervertebral disc cells in vitro (Fig. 1).
Human IVD cell culture in three dimensional alginate beads with low various concentrations of pamidronate (10-12, 10-9, 10-6, and 10-3 M) showed no significant increase in proteoglycan synthesis (Fig. 2).
In densitometric assay of RT-PCR, human IVD cell culture in three dimensional alginate beads with various dose of pamidronate (10-12, 10-9, 10-6, and 10-3 M) showed no statistically significant changes in mRNA expression of type I collagen, type II collagen, and aggrecan compared to control (Fig. 3).
Under the lower serum culture (1% FBS), human IVD cells in alginate beads showed upregulation of MMP-2, -3, -9, and -13 mRNA while no significant change in MMP-1 mRNA expression (Fig. 4).
Human IVD cells in lower serum and TNF-α (10 ng/mL) demonstrated also upregulations of MMP-2, -3, -9, and -13 mRNA. Human IVD cells with various doses of pamidronate (10-12, 10-9, 10-8, and 10-6 M) and lower serum and TNF-α (10 ng/mL) revealed partial down-regulation of MMPs. In detail, human IVD cells with above mentioned condition, showed down-regulation of MMP-1 mRNA (pamidronate 10-12), MMP-2 (pamidronate 10-9), MMP-3 (pamidronate10-8), MMP-9 (pamidronate 10-12,10-10, and 10-8), and MMP-13 (pamidronate 10-12, 10-10, and 10-8) (Fig. 5).
BPs have profound effect on skeletal tissue since they have inherent affinity to hydroxyapatite. Fundamental effect of BPs on skeletal tissue is inhibition of osteoclastic bone resorption by increasing apoptosis and decreasing cellular function of osteoclast, leading to maintenance of trabecular and cortical bone volume.[12] Hence BPs have been utilized in various skeletal conditions due to increased osteoclastic activity including postmenopausal osteoporosis, steroid induced osteoporosis, Paget's disease, tumoral bone disease, and particle induced bone resorption around metal implant.[171820] Recently, non-skeletal effects of BPs have been investigated in various tissues i.e., articular cartilage, chondrocyte.[1114192021] With regard to non-skeletal effect of BPs, it appears to be beneficial in terms of metabolism and maintenance of chondrogenic phenotype which include articular cartilage and IVD. IVDs are known to express chondrogenic phenotype and have similar functions like articular cartilage.
In this experimental study, in vitro effects of pamidronate (second generation nitrogen-containing BPs) were investigated using human IVD cells. The results showed that pamidronate exhibited no significant changes on cellular proliferation, proteoglycan synthesis, and chondrogenic phenotypycal expressions which comprise type II collagen and aggrecan mRNA. Also Pamidronate did not exert any cytotoxic effect on human IVD cells as evidence by sustained DNA and proteoglycan synthesis. Those findings imply pamidronate appears to maintain chondrogenic phenotypes at least, not down-regulate expressions of chondrogenic phenotype.
In lower serum condition (FBS 1%), there was significant upregulation of MMP-2, -3, -9, and -13 mRNA expression in human IVD cells. Contrary to that, human IVD cells under lower serum and TNF-α stimulation exhibited demonstrated down-regulation of MMPs in certain dose of pamidronate. Pamidronate with a dose of 10-12 M showed down-regulation of MMP-1, -9, and -13 mRNA, pamidronate with 10-8 M showed down-regulation of MMP-3, -9, and -13 mRNA, and pamidronate with 10-10 M demonstrated down-regulation of MMP-9, and -13 mRNA. Therefore lower dose of pamidronate (10-12 to 10-8 M) inhibited the expression of MMPs in IVD cell cultures with lower serum and TNF-α. Only exception was MMP-2 mRNA expression which was inhibited by pamidronate with a dose of 10-6 M. Synovial fibroblasts in response to TNF-α and interleukins produces cytokines which cause inflammation, angiogenesis, and cartilage degradation through matrix-degrading enzymes including MMP-13.[3334353637] Human IVD cells with response to TNF-α clearly demonstrated that increase in MMPs expression most importantly MMP-13 and those upregulations of MMPs mRNA expression were significantly abrogated with lower dose of pamidronate (10-12 to 10-8 M) in vitro. TNF-α plays an important role in inflammatory arthritis and IVD degeneration.[192122] TNF-α secreted from inflammatory cells and IVD cells triggers MMPs expression which ends up in vicious cycle of cell apoptosis and matrix degradation. The result of the current study demonstrated pamidronate can maintain chondrogenic phenotype and also down-regulated TNF-α induced expressions of MMP-1, -2, -3, -9, and -13. TNF-α blocker is now widely used in the treatment of inflammatory arthritis, sero-negative spondyloarthropathy, and in certain cases of IVD herniation.[38] Cartilageneous matrix protection of pamidronate by maintaining phenotypical expression and inhibiting expression of MMPs might render another beneficial and therapeutic importance in the treatment of IVD degeneration. The limitation of this study is primarily focused on the changes in gene expression and is insufficient in the expression of the protein i.e., MMPs and collagens. Also, it was not demonstrated how pamidronate inhibits TNF-α directly in this study. In the future, it is needed how pamidronate acts at the TNF-α block and inhibits the expression of MMPs in the protein level.
The results of our study correspond with the growing evidence that despite their apoptotic effects on osteoclasts and macrophages, BPs appear to have beneficial effects on other cells. It has been shown that BPs can enhance the differentiation and bone forming activities of osteoblasts [39] and also it has been demonstrated that BPs prevent steroid induced apoptosis of osteoblasts in vitro and in vivo. [40] Furthermore, recent results have clearly demonstrated that pamidronate has chondroprotective effect via maintaining chondrogenic phenotype expression and down-regulating MMPs expression.
In conclusion, pamidronate, nitrogen-containing second generation BPs, was safe in metabolism of IVD in vitro maintaining chondrogenic phenotype and matrix synthesis, and down-regulated TNF-α induced MMPs expression. Hence BPs can exert beneficial and therapeutic effects on IVD.
Figures and Tables
Fig. 1
Human intervertebral disc cells were cultured in three dimensional alginate beads with various concentration of pamidronate (10-12, 10-10, 10-8, and 10-6 M). DNA synthesis was analyzed with 3H-thymidine incorporation. Cultures with pamidronate showed no significant change in DNA synthesis compared to cultures without pamidronate, indicating pamidronate did render neither cellular proliferative nor cytotoxic effect on human Intervertebral disc cells in vitro. CPM, counts per min.

Fig. 2
Human intervertebral disc cells were cultured in three dimensional alginate beads with various concentration of pamidronate (10-12, 10-10, 10-8, and 10-6 M). Proteoglycan synthesis was analyzed with 35S-sulfate incorporation and normalized by deoxyribonucleic acid synthesis. Cultures with pamidronate demonstrated no significant increase in proteoglycan synthesis compared to control culture.

Fig. 3
In densitometric assay of reverse transcription-polymerase chain reaction, human intervertebral disc cell cultures in three dimensional alginate beads with various dose of pamidronate (10-12, 10-10, 10-8, and 10-6 M) showed no statistically significant changes in messenger RNA (mRNA) expression of type I collagen, type II collagen, and Aggrecan, compared to control. The mRNA expressions were normalized by beta-actin mRNA expression.

Fig. 4
Matrix metalloproteinase (MMP)-1, -2, -3, -9, and -13 messenger RNA (mRNA) expressions of human intervertebral disc cells under lower serum culture (1% fetal bovine serum). Cultures in lower serum showed 70% to 200% increase in MMP-2, -3, -9, and -13 while no change in expression of MMP-1 (*P<0.05). There were no significant changes in MMPs mRNA expression with given dose of pamidronate. FBS, fetal bovine serum.
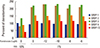
Fig. 5
Human intervertebral disc (IVD) cells in lower serum and tumor necrosis factor alpha (TNF-α; 10 ng/mL) demonstrated also upregulations of matrix metalloproteinase (MMP)-2, -3, -9, and -13 messenger ribo nucleic acid. Human IVD cells with various doses of pamidronate (10-12, 10-9, 10-8, and 10-6 M) and lower serum and TNF-α (10 ng/mL) reveled partial down-regulation of MMPs. FBS, fetal bovine serum; mRNA, messenger ribo nucleic acid.

Notes
References
1. Hughes DE, Wright KR, Uy HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995; 10:1478–1487.

2. Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999; 25:97–106.

3. Suri S, Mönkkönen J, Taskinen M, et al. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone. 2001; 29:336–343.

4. Alakangas A, Selander K, Mulari M, et al. Alendronate disturbs vesicular trafficking in osteoclasts. Calcif Tissue Int. 2002; 70:40–47.

5. Mayahara M, Sasaki T. Cellular mechanism of inhibition of osteoclastic resorption of bone and calcified cartilage by long-term pamidronate administration in ovariectomized mature rats. Anat Rec A Discov Mol Cell Evol Biol. 2003; 274:817–826.

6. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002; 112:281–289.

7. Millett PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002; 84-a:236–249.

9. Van Offel JF, Schuerwegh AJ, Bridts CH, et al. Effect of bisphosphonates on viability, proliferation, and dexamethasone-induced apoptosis of articular chondrocytes. Ann Rheum Dis. 2002; 61:925–928.

10. Garnero P, Christgau S, Delmas PD. The bisphosphonate zoledronate decreases type II collagen breakdown in patients with Paget's disease of bone. Bone. 2001; 28:461–464.

11. Podworny NV, Kandel RA, Renlund RC, et al. Partial chondroprotective effect of zoledronate in a rabbit model of inflammatory arthritis. J Rheumatol. 1999; 26:1972–1982.
12. Hardingham TE, Adams P. A method for the determination of hyaluronate in the presence of other glycosaminoglycans and its application to human intervertebral disc. Biochem J. 1976; 159:143–147.

13. Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987; 5:198–205.

14. Melrose J, Ghosh P, Taylor TK, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992; 10:665–676.

15. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976). 1995; 20:1307–1314.

16. Butler D, Trafimow JH, Andersson GB, et al. Discs degenerate before facets. Spine (Phila Pa 1976). 1990; 15:111–113.

17. Hiyama A, Sakai D, Risbud MV, et al. Enhancement of intervertebral disc cell senescence by WNT/beta-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010; 62:3036–3047.

18. Erwin WM, Islam D, Inman RD, et al. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011; 13:R215.

19. Wang M, Tang D, Shu B, et al. Conditional activation of beta-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012; 64:2611–2623.

20. Sato S, Kimura A, Ozdemir J, et al. The distinct role of the Runx proteins in chondrocyte differentiation and intervertebral disc degeneration: findings in murine models and in human disease. Arthritis Rheum. 2008; 58:2764–2775.

21. Wang H, Liu H, Zheng ZM, et al. Role of death receptor, mitochondrial and endoplasmic reticulum pathways in different stages of degenerative human lumbar disc. Apoptosis. 2011; 16:990–1003.

22. Pratsinis H, Constantinou V, Pavlakis K, et al. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012; 30:958–964.

23. Studer RK, Vo N, Sowa G, et al. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-alpha. Spine (Phila Pa 1976). 2011; 36:593–599.
24. Moon HJ, Kim JH, Lee HS, et al. Annulus fibrosus cells interact with neuron-like cells to modulate production of growth factors and cytokines in symptomatic disc degeneration. Spine (Phila Pa 1976). 2012; 37:2–9.

25. Melrose J, Shu C, Young C, et al. Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Phila Pa 1976). 2012; 37:18–25.

26. Yurube T, Takada T, Suzuki T, et al. Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther. 2012; 14:R51.

27. Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012; 1824:133–145.

28. Kim MS, Kim JH, Lee MR, et al. Effects of alendronate on a disintegrin and metalloproteinase with thrombospondin motifs expression in the developing epiphyseal cartilage in rats. Anat Histol Embryol. 2009; 38:154–160.

29. Teronen O, Heikkilä P, Konttinen YT, et al. MMP inhibition and downregulation by bisphosphonates. Ann N Y Acad Sci. 1999; 878:453–465.

30. Bone HG, Hosking D, Devogelaer JP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004; 350:1189–1199.

31. Chelberg MK, Banks GM, Geiger DF, et al. Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat. 1995; 186(Pt 1):43–53.
32. Maldonado BA, Oegema TR Jr. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992; 10:677–690.

33. Verbruggen A, De Clerck LS, Bridts CH, et al. Influence of blood and synovial fluid immune complexes of patients with rheumatoid arthritis on production of nitric oxide and growth and viability of chondrocytes. J Rheumatol. 2000; 27:35–40.
34. Igarashi H, Kouro T, Yokota T, et al. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci U S A. 2001; 98:15131–15136.

35. Lemon WJ, Bernert H, Sun H, et al. Identification of candidate lung cancer susceptibility genes in mouse using oligonucleotide arrays. J Med Genet. 2002; 39:644–655.

36. Brennan FM, Smith NM, Owen S, et al. Resting CD4+ effector memory T cells are precursors of bystander-activated effectors: a surrogate model of rheumatoid arthritis synovial T-cell function. Arthritis Res Ther. 2008; 10:R36.

37. Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008; 118:3537–3545.

38. Williams LM, Lali F, Willetts K, et al. Rac mediates TNF-induced cytokine production via modulation of NF-kappaB. Mol Immunol. 2008; 45:2446–2454.
39. Reinholz GG, Getz B, Pederson L, et al. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000; 60:6001–6007.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download