Abstract
Background
Epidemiology studies suggest that oral bisphosphonate may increase the risk of esophageal cancer. The present study aimed to investigate the association between exposure of oral bisphosphonate and risk of esophageal cancer.
Methods
Using the nationwide medical claim database in South Korea, 2,167,955 subjects, who initiated osteoporosis treatment (oral bisphosphonate, intravenous bisphosphonate or raloxifene) or performed dual energy X-ray absorptiometry (DXA) between 2008 and 2012, were analyzed. Diagnosis of esophageal cancer was estimated from medical claim database. Standardized incidence ratio (SIR) was estimated by comparing with incidence in the general population. Cox proportional hazards modeling was used to investigate age-adjusted hazard ratio (aHR) of esophageal cancer.
Results
The present study included oral bisphosphonate group (N=1,435,846), comparator group 1 (intravenous bisphosphonate or raloxifene, N=78,363) and comparator group 2 (DXA, N=653,746). Mean age was 65.6±8.8 years and mean observation duration was 30.9±17.7 months. During 5,503,688 patient-years, 205 esophageal cancer incidences were observed. The annual incidence of esophageal cancer was 3.88, 4.21, and 3.30 for oral bisphosphonate group, comparator group 1 and comparator group 2, respectively. SIR of esophageal cancer was 1.24, 1.38, and 1.40 for oral bisphosphonate group, comparator group 1 and comparator group 2, respectively. Esophageal cancer risk of oral bisphosphonate group was not significantly different from comparator group 1 and comparator group 2 (aHR 0.87; 95% confidence interval [CI] 0.39-1.98 and aHR 0.94; 95% CI 0.68-1.30, respectively).
Osteoporosis and osteoporotic fracture are prevalent diseases, and bisphosphonates are widely used for osteoporosis treatment.[1,2,3,4] Oral bisphosphonate cause dyspepsia and inflammatory damage to esophagus. This chemical irritation has been suggested as a mechanistic link between oral bisphosphonate use and risk of esophageal cancer.
Between the initiation of alendronate marketing in 1994 and 2008, US Food and Drug Administration (FDA) received reports of 23 patients who were diagnosed with esophageal cancer with alendronate as the suspect drug or the concomitant drug.[5] There has been report of 31 esophageal cancer patients after using alendronate (the suspect drug for 21 patients) from Europe and Japan.[5] And it has been suggested that studies should include oral bisphosphonate as a possible risk factor for esophageal cancer.[5]
After the FDA reports, numerous studies investigated the association between risk of esophageal cancer and use of oral bisphosphonate. A report from UK primary care cohort concluded that the risk of esophageal cancer increased in patients with oral bisphosphonate compared with no prescriptions (relative risk of 1.30), rising to more than twofold increase for more than three years' use.[6] However, there has been inconsistency regarding this issue even using the same UK primary care cohort database.[7,8] Due to the relative rarity of esophageal cancer and the lack of large scale database, the confidence intervals (CIs) were wide to draw a clinically significant conclusion.
The aim of this study was to investigate the association between use of oral bisphosphonate and the risk of esophageal cancer using a nationwide claim database in Korea.
The present study was a retrospective study of the nationwide claims from the Health Insurance Review and Assessment (HIRA) service of South Korea. The HIRA database covered over 99.9% of all medical claims in South Korea. The HIRA database includes diagnosis records, procedure records, prescription records, and demographic information. [3,9,10,11,12,13] The study protocol was approved by the Institute Review Board of Chungbuk National University Hospital. The age and gender distribution of Korean general population was obtained from the Korean Statistical Information Service.
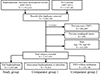
We examined female subjects (age 50 to 84 years) who had medical claim records of bisphosphonate (alendronate, risedronate or ibandronate) and raloxifene prescription medications or DXA from 2007 to 2012. The first medical claim record date was set as the index date. We included subjects who initiated the medication without previous exposure within one year by excluding subjects who had prescription records of the medication in 2007. We also excluded subjects who had esophageal cancer diagnosis before 2008 by excluding subjects who had medical claim records of esophageal cancer in 2007 (one in-patient record or more than 3 out-patient records).
Based on the medical claim record, subjects were categorized into three groups. Oral bisphosphonate group included subjects who initiated oral bisphosphonate (alendronate, risedronate or ibandronate). Comparator group 1 included subjects who initiated intravenous bisphosphonate or raloxifene (without any oral bisphosphonate prescription). Comparator group 2 included subjects who had DXA but did not have any prescription of bisphosphonate or raloxifene.
Esophageal cancer outcomes were identified on the basis of insurance claim data using selected International Classification of Diseases, Tenth Revision (ICD-10) codes. The first claim data of esophageal cancer as the major diagnosis code in in-patient records was included as esophageal incidence.
Esophageal cancer incidence was analyzed during the observation period and incidence rates with 95% CIs were calculated by using Poisson distribution. The standardized incidence ratio (SIR), was calculated as observed incidence divided by expected incidence for each age group. Oral bisphosphonate group was compared to comparator group 1 and 2. Adjusted hazard ratios (aHR) adjusting for age were calculated by using cox proportional hazard model. Statistical threshold of P<0.05 was considered as significant.
From the national claims database between 2008 and 2012,2,167,955 eligible subjects with bisphosphonate or raloxifene medications or DXA exam were identified (Fig. 1).
Baseline characteristics are shown in Table 1. Mean age was 65.6 years and mean observation duration was 30.9 months.
Esophageal cancer incidence and adjusted HR are shown in Table 2. For 1,435,846 oral bisphosphonate users, 147 esophageal cancer incidences were observed among 3,785,045 person-years observation. For 78,363 comparator group1 subjects, 6 esophageal cancer incidences were observed among 142,544 person-years observation. For 653,746 comparator group 2 subjects, 52 esophageal cancer incidences were observed among 1,576,099 person-years observation. The crude incidence rate (cases per 100,000 person-years) of esophageal cancer was 3.88 for oral bisphosphonate users, 4.21 for comparator group 1 and 3.30 for comparator group 2. Compared with general population, SIR was 1.24 (1.08-1.42, P=0.007) for oral bisphosphonate group, 1.38 (0.60-2.73, P=0.269) for comparator group 1 and 1.40 (1.09-1.76, P=0.013) for comparator group 2.
In this nationwide study, we found that the use oral bisphosphonate was not associated with increased risk of esophageal cancer. The oral bisphosphonate group had similar esophageal cancer incidence compared with comparator groups.
There has been increasing concern regarding oral bisphosphonates and the risk of esophageal cancer.[14,15] However, related studies have yielded controversial results depending on study population and study design. Green et al.[6] investigated a nested case control study using UK General Practice Research Database (GPRD) reporting increased risk of esophageal cancer in bisphosphonate users (relative risk 1.30, 95% CI 1.02-1.66). Another study by Wright et al.[16] using GPRD also concluded that bisphosphonate was associated with increased risk of esophageal cancer (odds ratio 1.54, 95% CI 1.2-1.88). However, Cardwell et al.[7] performed retrospective cohort study using the GPRD and concluded that no evidence of an increase in the risk for gastric and esophageal cancer in bisphosphonate users (HR 0.96, 95% CI 0.74-1.49). A retrospective cohort study from Denmark by Vestergaard [17] reported that bisphosphonate was associated with increased risk of esophageal cancer (relative risk 2.10, 95% CI 1.01-4.35). Two recent meta-analysis papers concluded that bisphosphonate is not associated with risk of esophageal cancer.[18,19] One case-control study and one cohort study using nationwide health insurance database in Taiwan also concluded that bisphosphonate is not associated with risk of esophageal cancer.[20,21]
The SIR of oral bisphosphonate group was 1.24 (95% CI 1.08-1.42, P=0.007). This result could lead to conclusion that oral bisphosphonate use is associated with increased risk of esophageal cancer. However, the SIRs of comparator groups (osteoporosis patient using intravenous bisphosphonate or raloxifene or subjects who performed DXA test but did not receive osteoporosis medications) were higher than that of oral bisphosphonate group; 1.38 (95% CI 0.60-2.73, P=0.269) and 1.40 (95% CI 1.09-1.76, P=0.013), respectively. These results indicate that oral bisphosphonate per se is not associated with risk of esophageal cancer and other factors associated with osteoporosis treatment (regardless of oral bisphosphonate or other treatment) are associated with risk of esophageal cancer. We believe these findings illustrate the pitfalls of epidemiology study design, including SIR analysis. There could be common risk factor affecting both osteoporosis and esophageal cancer, such as smoking. If osteoporosis per se is associated with increased risk of esophageal cancer, due to these possible confounding factors, comparing bisphosphonate users to non-users (or health control) could be misleading and could lead to overestimation of outcome risk. The increased SIR of oral bisphosphonate users (compared to general population) could also be due to more frequent medical utilization among oral bisphosphonate users (such as endoscopy).
There are several strengths of the present study. Firstly, to our knowledge, the present study is the largest retrospective cohort study investigating the association between oral bisphosphonate use and risk of esophageal cancer. The present study investigated 147 esophageal cancer cases from 1,435,846 oral bisphosphonate users, whereas previous retrospective cohort studies investigated fewer subjects (79 esophageal cancer cases from 41,826 bisphosphonate users,[7] 49 esophageal cancer cases from 92,975 bisphosphonate users,[17] 3 esophageal cancer cases from 5,624 bisphosphonate users).[21] Secondly, the present study investigated only female subjects. Considering the heterogeneity between male and female regarding esophageal cancer, this study design increases the homogeneity and the internal validity of the interpretation. Thirdly, the present study used two comparators (other osteoporosis treatment group and non-treated osteoporosis group) to avoid possible biases from study design. Thirdly, the present study analyzed SIR for the oral bisphosphonate group and 2 comparator groups to avoid the pitfall of using SIR. Fourthly, the present study investigated the nationwide database reflecting the real-world clinical practice.
The present study has several limitations. Firstly, possible confounders associated with esophageal cancer, such as smoking, were not assessed.[12] Secondly, drug exposure dose or non-adherent use could not be investigated adequately due to the reimbursement policy in Korea. Thirdly, the outcome data was based on insurance claim database and this limitation may under- or over-estimate the esophageal cancer incidence. Fourthly, due to the study design, the present study is prone to detection bias (such as difference in medical utilization frequency or endoscopy frequency).
In conclusion, we found no evidence for a substantial increase in esophageal cancer risk among oral bisphosphonate users in real clinical practice using large scale nationwide database.
Figures and Tables
Fig. 2
Kaplan-Meier curves comparing time to esophageal cancer in the oral bisphosphonate and comparator groups. Oral BP, oral bisphosphonate; IV BP, intravenous bisphosphonate; Ralo, Raloxifene; DXA, dual energy X-ray absorptiometry

References
1. Shin CS, Choi HJ, Kim MJ, et al. Prevalence and risk factors of osteoporosis in Korea: a community-based cohort study with lumbar spine and hip bone mineral density. Bone. 2010; 47:378–387.

2. Shin CS, Kim MJ, Shim SM, et al. The prevalence and risk factors of vertebral fractures in Korea. J Bone Miner Metab. 2012; 30:183–192.

3. Lee YK, Ha YC, Choi HJ, et al. Bisphosphonate use and subsequent hip fracture in South Korea. Osteoporos Int. 2013; 24:2887–2892.

4. Chung DJ, Choi HJ, Chung YS, et al. The prevalence and risk factors of vertebral fractures in Korean patients with type 2 diabetes. J Bone Miner Metab. 2013; 31:161–168.

5. Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009; 360:89–90.

6. Green J, Czanner G, Reeves G, et al. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010; 341:c4444.

7. Cardwell CR, Abnet CC, Cantwell MM, et al. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010; 304:657–663.

8. Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to bisphosphonates and risk of gastrointestinal cancers: series of nested case-control studies with QResearch and CPRD data. BMJ. 2013; 346:f114.

9. Kim SH, Ko YB, Lee YK, et al. National utilization of calcium supplements in patients with osteoporotic hip fracture in Korea. J Bone Metab. 2013; 20:99–103.

10. Seo GH, Lee YK, Ha YC. Risk of hip fractures in men with alpha-blockers: a nationwide study base on claim registry. J Bone Metab. 2015; 22:29–32.

11. Park C, Jang S, Lee A, et al. Incidence and mortality after proximal humerus fractures over 50 years of age in South Korea: national claim data from 2008 to 2012. J Bone Metab. 2015; 22:17–21.

12. Choi HJ, Park C, Lee YK, et al. Risk of fractures in subjects with antihypertensive medications: A nationwide claim study. Int J Cardiol. 2015; 184:62–67.

13. Choi HJ, Shin CS, Ha YC, et al. Burden of osteoporosis in adults in Korea: a national health insurance database study. J Bone Miner Metab. 2012; 30:54–58.

14. Ha YC, Lee YK, Lim YT, et al. Physicians' attitudes to contemporary issues on osteoporosis management in Korea. J Bone Metab. 2014; 21:143–149.

15. Kong SY, Kim DY, Han EJ, et al. Effects of a 'drug holiday' on bone mineral density and bone turnover marker during bisphosphonate therapy. J Bone Metab. 2013; 20:31–35.

16. Wright E, Schofield PT, Seed P, et al. Bisphosphonates and risk of upper gastrointestinal cancer--a case control study using the General Practice Research Database (GPRD). PLoS One. 2012; 7:e47616.
17. Vestergaard P. Occurrence of gastrointestinal cancer in users of bisphosphonates and other antiresorptive drugs against osteoporosis. Calcif Tissue Int. 2011; 89:434–441.

18. Sun K, Liu JM, Sun HX, et al. Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies. Osteoporos Int. 2013; 24:279–286.

19. Oh YH, Yoon C, Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: meta-analysis of observational studies. World J Gastroenterol. 2012; 18:5779–5788.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download