Abstract
Background
Osteoclasts are differentiated from monocytes/macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL). Croton pycnanthus Benth. (CPB) is a herbal plant that belongs to Euphorbiaceae family. The aim of this study was to investigate the effects of CPB on osteoclastogenesis and RANKL-dependent signaling pathways.
Methods
Methanol extract of CPB was obtained from International Biological Material Research Center. Osteoclast differentiation was achieved by culturing mouse bone marrow-derived macrophages (BMMs) with M-CSF and RANKL. Osteoclast numbers were evaluated by counting multinuclear cells positive for tartrate-resistant acid phosphatase (TRAP). mRNA and protein levels were analyzed by real-time polymerase chain reaction (PCR) and Western blotting, respectively. The activation of signaling molecules were assessed after acute stimulation of cells with high dose of RANKL by Western blotting with phospho-specific antibodies.
Results
CPB reduced the generation of TRAP-positive multinucleated cells and the activation of mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways. The induction of the expression of c-Fos, nuclear factor-activated T cells c1 (NFATc1) and dendritic cell-specific transmembrane protein (DC-STAMP) by RANKL was also suppressed.
Osteoclasts are stemmed from monocyte/macrophage lineage of hematopoietic cells in response to the macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL).[1,2,3] M-CSF is an important factor for the proliferation and survival of the cells during differentiation progression and for the up-regulation of the expression of receptor activator of NF-κB (RANK), the receptor for RANKL, in precursor cells. RANKL is the osteoclast differentiation factor that drives and governs the differentiation per se.
During the differentiation, two transcription factors, c-Fos and nuclear factor-activated T cells c1 (NFATc1), play key roles for the expression of osteoclast marker genes.[4] Accordingly, both c-Fos deficient and NFATc1 deficient mice showed increased bone volume due to defective osteoclastogenesis.[5,6] The NFATc1 transcription factor has an intriguing feature of auto-amplification, in which its initial activation leads to a positive feedback for its own transcription.[7]
Upon binding of RANKL to its receptor RANK, many intracellular signaling pathways are stimulated.[8] Those pathways include the mitogen-activated protein kinase (MAPK) pathway. All the major members of MAPKs, extracellular signal-regulated kinase (ERK), c-Jun-N-terminal kinase (JNK), and p38, have been reported to be activated by RANKL. The activated ERK can phosphorylates and subsequently activates the c-Fos transcription factor. Other signaling molecules stimulated by RANKL include Akt and NF-κB. The activation of Akt is important for survival of osteoclasts. The activation of NF-κB is mediated by the phosphorylation of inhibitor of kappa B kinase (IKK) that phosphorylates IκB for subsequent ubiquitination and degradation of inhibitory κB, which leads to release and translocation of NF-κB to the nucleus. Alternatively, the phosphorylation of p65 NF-κB increases the transcriptional activity of NF-κB. For the activation of NFATc1, the intracellular calcium concentration is elevated in response to RANKL and a co-stimulatory signal from Ig-like receptor.[9] Calcium then binds calcineurin that dephosphorylates NFATc1, allowing nuclear translocation of NFATc1 for transcriptional activity.
Many plants have been used and developed as medicines for several diseases. International Biological Material Research Center (IBMRC, http://www.ibmrc.re.kr) in Korea has provided extracts of thousands of plants from several countries to researchers to help develop new drugs from natural resources. We performed cell-based screening with several plant extracts obtained from IBMRC to find new agents with potential therapeutic effects on osteoporosis and other osteolytic diseases. Among them, methanol extract of Croton pycnanthus Benth. (CPB, PBEC10101) showed inhibitory effects on osteoclast differentiation without cytotoxicity. CPB is a family of Euphorbiaceae which grows naturally in Mosquerillo, Ecuador. We further investigated the effects of CPB on RANKL-dependent signaling pathways to find a molecular mechanism for the anti-osteoclastogenic activity of CPB.
SYBR PCR Master Mix was purchased from Kapa Biosystems (Boston, MA, USA). Anti-mouse and anti-rabbit IgG-conjugated HRP and anti-mouse actin antibodies were purchased from Sigma-Aldrich (St Louis, MO, USA). Antibodies against ERK, phospho-ERK, JNK, phospho-JNK, p38, phospho-p38, Akt, phospho-Akt, p65, phospho-p65, IKK, and phospho-IKK were obtained from Cell Signaling Technology (Cambridge, MA, USA).
Methanol extract of CPB was obtained from IBMRC. Briefly, CPB was extracted by 3 day sonication (15 min sonication/2 hr stop, 10 times/day) in 99.99% methyl alcohol (high-performance liquid chromatography [HPLC] grade) at 45℃. After filtration, extract was concentrated by rotary evaporator (N-1000SWD) at 45℃ and dried using a speed vacuum concentrator (Modul 4080C, Biotron Inc., Bucheon, Korea) at 45℃ and -70℃ for 24 hr. Final extract was stored at -4℃. Extract was dissolved in dimethyl sulfoxide (Sigma Aldrich) and then diluted in PBS.
Mouse bone marrow cells were isolated from the bone marrow of femurs and tibiae of 5 week-old mice (Orient Bio, Seongnam, Korea). Bone marrow cells were cultured in alpha minimum essential medium (α-MEM; JBI, Daegu, Korea) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) for one day. Nonadherent cells were collected and further cultured in the presence of 30 ng/mL M-CSF for 3 days. Cells at this stage were considered bone marrow-derived macrophages (BMMs) and used as osteoclast precursors. For osteoclast differentiation, BMMs were cultured in the presence of M-CSF (30 ng/mL) and RANKL (100 ng/mL).
BMMs were plated in 96 well plates at the density of 1×104 cells per well. Cells were incubated with M-CSF and RANKL in the absence or presence of CPB at various concentrations. After 4 days of incubation, cells were fixed with 3.7% formaldehyde and permeabilized with 0.1% Triton X-100. Cells were stained for TRAP activity using Leukocyte Acid Phosphatase Kit (Sigma, Cat. No. 387A-1KT) following the manufacturer's protocol. TRAP-positive cells were quantified using the Olympus23 light microscope (Tokyo, Japan).
Total RNA was extracted from cultured cells using TRIZOL (Invitrogen, Carlsbad, CA, USA). Three µg of RNA was reverse transcribed to the complementary DNA using a reverse transcriptase (Thermo Scientific, Waltham, MA, USA). Twenty ng of complementary DNA (total volume of 20 µL) was used for DNA amplification. Real-time PCR was performed with SYBR PCR Master Mix using ABI7500 (Life Technologies, Carlsbad, CA, USA). The amount of mRNA was normalized using actin levels.
For detection of transcription factors related to osteoclast differentiation, 2×105 BMM cells were seeded in 6-well plates and treated with M-CSF (30 ng/mL) and RANKL (100 ng/mL) in the absence or presence of CPB (10 µg/mL). For signal transduction study, 1×106 BMM cells were plated in 60 mm dish and starved for 6 hr in α-MEM without FBS. After 3 hr of starvation, CPB (10 µg/mL) was added. At completion of starvation, cells were treated with RANKL (500 ng/mL) for 5-30 min. Cells were washed with PBS and lysed using a lysis buffer containing 10 mM Tris pH 7.2, 150 mM NaCl, 5 mM EDTA, 1 mM NaF, 2.5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 µg/mL leupeptin, 1 µg/mL aprotinin, 1% Triton X-100, 0.1% SDS, and 1% deoxycholate. 30 µg of lysate protein samples were loaded to SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blotted with various primary antibodies overnight at 4℃ and subsequently with secondary antibodies for 1 hr at room temperature. The generated immune complexes were detected using enhanced chemiluminescence reagents.
To investigate whether CBP regulates osteoclast differentiation, mouse primary BMMs were cultured with M-CSF and RANKL in the absence or presence of CPB at various concentrations. CPB had inhibitory effects on osteoclast differentiation as shown by the TRAP-staining assay (Fig. 1A). With 0.01 µg/mL of CPB, the number of TRAP-positive multinucleated cells (osteoclasts) was diminished to 30% of the control group. The number of osteoclasts generated was reduced by about 90% at 10 µg/mL of CPB (Fig. 1B). These results indicate that CBP has an anti-osteoclastogenic activity. To test the possibility that the reduction in osteoclast generation might have been attributed by the potential cytotoxicity of CPB, we carried out a cytotoxicity assay with cell counting kit (CCK) reagents (Itsbio, Seoul, Korea). CPB did not have any effect on viability of BMMs at concentrations up to 20 µg/mL (Fig. 1C).
There are several important molecular mediators of osteoclast differentiation and function. c-Fos and NFATc1 are main transcription factors crucial for the induction of gene expression associated with osteoclastogenesis. These two transcription factors are increased by RANKL during osteoclast differentiation. When BMMs were treated with 10 µg/mL of CPB, the induction of both c-Fos and NFATc1 mRNA expression by RANKL was attenuated compared to the control group (Fig. 2A, B). DC-STAMP has been shown to be involved in the cell fusion during osteoclastogenesis and in the bone resorptive function of osteoclasts.[10] The mRNA level of DC-STAMP was greatly increased by RANKL in the absence of CPB whereas the presence of CPB suppressed the induction of DC-STAMP by RANKL (Fig. 2C). These results support the anti-osteoclastogenic activity of CPB in addition to the results of TRAP-staining experiments.
We next examined the effect of CPB on protein levels of c-Fos and NFATc1 during osteoclast differentiation by Western blotting analyses. As shown in Fig. 3, the protein level of c-Fos was dramatically increased and reached a peak at day 1, and later showed a modestly increase at day 2 during incubation with RANKL. This increase in c-Fos protein by RANKL was inhibited by the addition of CPB in the culture. Similarly, RANKL elevated the protein level of NFATc1 at day 1, further increasing the level at day 2. CPB almost completely suppressed the induction of NFATc1 protein expression by RANKL (Fig. 3).
RANKL stimulates several signaling pathways that involve MAPKs (ERK, JNK, and p38), PI3K, Akt, and NF-κB. To investigate which signaling pathways are affected by CPB, we measured the phosphorylated active form of signaling molecules using specific antibodies. Consistent with previous reports, RANKL stimulated the phosphorylation of ERK, JNK, and p38 within 30 min (Fig. 4). The addition of CPB significantly reduced the extent of increases in phospho-ERK, phospho-JNK, and phosph-p38 levels by RANKL (Fig. 4). These results suggest that the suppression of RANKL signaling to MAPKs is a part of mechanism by which CPB inhibits osteoclast differentiation.
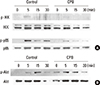
We next assessed the effect of CPB on the stimulation of NF-κB and Akt signaling pathways by RANKL. RANKL weakly stimulated the phosphorylation of IKK and CPB abolished the IKK activation (Fig. 5A). RANKL also increased the phosphorylated form of p65 NF-κB. This activation of p65 by RANKL was greatly inhibited by the presence of CPB (Fig. 5A). The activation of Akt by RANKL was also observed in the Western blotting experiments with a phopho-Akt antibody (Fig. 5B). Unlike IKK and p65, the activation of Akt by RANKL was not suppressed by CPB (Fig. 5B). Taken together, the inhibition of osteoclast differentiation by CPB is likely to be caused by suppression of the MAPK and NF-κB, but not Akt, signaling pathways of RANK.
In this paper, we screened several plant extracts obtained from IBMRC to discover potential therapeutic agents with anti-osteoclastogenic activity. Among them, CPB showed inhibitory effects on osteoclast differentiation without cytotoxicity. CPB suppressed the induction of expression of c-Fos and NFATc1 by RANKL at both mRNA and protein levels. CPB also suppressed the RANKL-dependent activation of MAPK and NF-κB, but not Akt, signaling pathways.
The significance of RANK signaling in osteoclast differentiation has been well studied. RANK deficient mice showed osteopetrotic phenotype, which resulted from the failure of osteoclast differentiation.[11] RANKL deficient mice are also well characterized by their osteopetrotic morphology.[12] Therefore, it is important to focus on the regulation of RANKL-induced signaling pathways to control osteoclast differentiation.
RANK signaling promotes osteoclast differentiation through the MAPK and NF-κB pathways and osteoclast survival through the Akt pathway.[8] ERK that is stimulated by the Raf and MAPK kinase (MEK)1/2 axis eventually activates c-Fos while JNK activates c-Jun and activator protein-1 (AP-1). c-Fos and AP-1 interact each other and induce the expression of osteoclast marker genes. In case of p38, it activates another transcription factor microphthalmia-associated transcription factor (MITF), which also contributes to the expression of genes associated with osteoclastogenesis.[13,14] CPB suppressed all three MAPK signaling pathways (Fig. 4). The importance of NF-κB signaling in osteoclast differentiation has been well studied. NF-κB p50/p52 double-deficient mice have osteopetrotic phenotype, which caused by defects in osteoclastogenesis.[15] RANKL stimulation activates IKK and eventually elevates the transcription activity of p50, p52, and p65.[16] In our study, CPB effectively reduced the phosphorylation of IKK and p65 NF-κB (Fig. 5A). Therefore, it appears that the reduced osteoclastogenesis by CPB is achieved by its interference with the activation of NF-κB as well as MAPK signaling pathways by RANKL.
In many studies including our current study, the expression of c-Fos and NFATc1 was elevated by RANKL both at mRNA and protein levels. While the explanation for the up-regulation of c-Fos mRNA by RANKL is elusive, the RANKL induction of NFATc1 mRNA has been suggested to be mediated by c-Fos and NF-κB. NFATc1 protein in turn binds the promoter of its own gene and further stimulates NFATc1 mRNA expression.[7] CPB significantly reduced the levels of mRNA and protein of c-Fos and NFATc1, suggesting that CPB controlled the expression of these transcription factors at the transcriptional level. As the activation of NFATc1 transcription factor requires calcium signaling, examining the effect of CPB on calcium oscillation during osteoclast differentiation may be an intriguing future investigation.
Akt has been known for its ability to regulate survival of many types of cells including differentiated osteoclasts.[17] CPB did not affect to the activation of Akt signaling pathway by RANKL (Fig. 5B). It is consistent with the results of cytotoxicity assay in which CPB did not alter the viability of BMMs. Therefore, the negative regulation of CPB on osteoclast differentiation is not likely to be caused by decreased proliferation of BMMs or survival of differentiating osteoclasts.
Multinucleation is a distinct feature of osteoclasts and make mature osteoclasts easily distinguishable from their precursors. The cell fusion process for multinucleation during osteoclastogenesis has been suggested to be mainly mediated by DC-STAMP.[18] The expression of DC-STAMP mRNA was reduced by CPB in our study (Fig. 2C). DC-STAMP deficient mice showed increased bone mass.[10] In Paget's disease, which shows increased bone resorption, osteoclasts have much more nuclei than those of normal ones.[19] These reports therefore suggest that the extent of multinucleation may be, to a certain level, proportional to the proficiency of osteoclasts and that DC-STAMP level may have a correlation with the bone-resorbing activity of osteoclasts.
Various types of plant extracts have been developed as medicine. For example, Ayahuasca which grows naturally in Ecuador has been developed to a medicine, Da Vine, for cardiovascular disease.[20] Another example is Maca Lepidium meyenii from Peru that has antiviral activity.[21] Turmeric curcuma longa from India, applied as a salve, reduces inflammatory response.[22,23] As many government and enterprises have eyes on patents for plant medicine, it may be a useful strategy to screen extracts of plants from a broad range of countries to find components that have anti-osteolytic or pro-osteoblastic activity.
Further investigations are clearly required to develop CPB as a therapeutic agent. Experiments to validate the in vivo efficacy of CPB as well as identification of the components in CPB that is responsible for the anti-osteoclastogenic activity should be performed in future studies.
Figures and Tables
Fig. 1
Croton pycnanthus Benth. (CPB) decreased the number of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells. (A, B) 1×104 bone marrow-derived macrophages (BMM) cells were plated in 96 well plates and incubated with macrophage colony-stimulating factor (M-CSF; 30 ng/mL) and receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL; 100 ng/mL) in the absence or presence of CPB. After 4 days of incubation, cells were fixed with 3.7% formaldehyde and permeabilized with 0.1% Triton X-100 followed by TRAP-staining. TRAP-positive multinucleated cells were quantified using the Olympus23 light microscope. (C) 1×104 BMM cells were plated in 96 well plates and incubated with M-CSF (30 ng/mL) in the absence or presence of CPB. After 2 and 24 hr incubation, cell counting kit (CCK) reagents were added and absorbance at 450 nm was measured. TRAP, tartrate-resistant acid phosphatase; MNC, multinucleated cells.

Fig. 2
Croton pycnanthus Benth. (CPB) diminished the mRNA expression levels of c-Fos, nuclear factor-activated T cells c1 (NFATc1) and dendritic cell-specific transmembrane protein (DC-STAMP). 2×105 bone marrow-derived macrophages (BMM) cells were plated in 6 well plates and incubated with macrophage colony-stimulating factor (M-CSF; 30 ng/mL) and receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL; 100 ng/mL) in the absence or presence of CPB (10 µg/mL). After 1 and 2 day incubation, RNA was extracted and 3 µg of RNA was reverse-transcribed to the complementary DNA. Complementary DNA of c-Fos (A), NFATc1 (B), and DC-STAMP (C) were amplified by real-time polymerase chain reaction (PCR) and normalized to actin levels. CPB, Croton pycnanthus Benth.; NFATc1, nuclear factor-activated T cells c1; DC-STAMP, dendritic cell-specific transmembrane protein.

Fig. 3
Croton pycnanthus Benth. (CPB) decreased the c-Fos and nuclear factor-activated T cells c1 (NFATc1) protein levels. 2×105 bone marrow-derived macrophages (BMM) cells were seeded in 6-well plates and treated with macrophage colony-stimulating factor (M-CSF; 30 ng/mL) and receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL; 100 ng/mL) in the absence or presence of CPB (10 µg/mL). Thirty µg of protein samples were loaded to the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to the nitrocellulose membranes. Membranes were blotted with c-Fos, NFATc1 and actin primary antibodies for overnight at 4℃ and then with secondary antibodies for 1 hr at room temperature before detection with chemiluminescence reagents. CPB, Croton pycnanthus Benth.; NFATc1, nuclear factor-activated T cells c1.
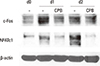
Fig. 4
Croton pycnanthus Benth. (CPB) suppressed the receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL)-induced mitogen-activated protein kinase (MAPK) activation. 1×106 bone marrow-derived macrophages (BMM) cells were plated in 60 mm dish. After 3 hr of starvation, CPB (10 µg/mL) was added. After further incubation for another 3 hr, cells were treated with RANKL (500 ng/mL) for 5-30 min and subjected to Western blotting with indicated antibodies. CPB, Croton pycnanthus Benth.; ERK, extracellular signal-regulated kinase; JNK, c-Jun-N-terminal kinase.
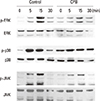
Fig. 5
Croton pycnanthus Benth. (CPB) suppressed the activation of nuclear factor-kappa B (NF-κB) pathway, but did not affect the Akt pathway. 1×106 bone marrow-derived macrophages (BMM) cells were plated in 60 mm dish. After 3 hr of starvation, CPB (10 µg/mL) was added. After further incubation for another 3 hr, cells were treated with receptor activator of NF-κB ligand (RANKL; 500 ng/mL) for 5-30 min and subjected to Western blotting analyses. Antibodies against p65, phospho-p65, inhibitor of kappaB kinase (IKK), and phospho-IKK were used to examine the NF-kB pathway (A), and antibodies against Akt and phospho-Akt were used to assess the Akt pathway (B). CPB, Croton pycnanthus Benth.; IKK, inhibitor of kappaB kinase.
References
1. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176.

2. Fuller K, Wong B, Fox S, et al. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998; 188:997–1001.

3. Miyamoto T, Ohneda O, Arai F, et al. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001; 98:2544–2554.

4. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.

5. Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005; 208:126–140.

6. Aliprantis AO, Ueki Y, Sulyanto R, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008; 118:3775–3789.

7. Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005; 202:1261–1269.

8. Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003; 305:211–214.

9. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7:292–304.

10. Yagi M, Miyamoto T, Sawatani Y, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005; 202:345–351.

11. Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999; 13:2412–2424.

12. Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999; 397:315–323.

13. Jimi E, Akiyama S, Tsurukai T, et al. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol. 1999; 163:434–442.
14. Lee SE, Woo KM, Kim SY, et al. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002; 30:71–77.

15. Iotsova V, Caamaño J, Loy J, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997; 3:1285–1289.
16. Wei S, Teitelbaum SL, Wang MW, et al. Receptor activator of nuclear factor-kappa b ligand activates nuclear factor-kappa b in osteoclast precursors. Endocrinology. 2001; 142:1290–1295.

17. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999; 13:2905–2927.

18. Zhang C, Dou CE, Xu J, et al. DC-STAMP, the key fusion-mediating molecule in osteoclastogenesis. J Cell Physiol. 2014; 229:1330–1335.

20. Riba J, Valle M, Urbano G, et al. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther. 2003; 306:73–83.

21. Del Valle Mendoza J, Pumarola T, Gonzales LA, et al. Antiviral activity of maca (Lepidium meyenii) against human influenza virus. Asian Pac J Trop Med. 2014; 7S1:S415–S420.

22. Arora RB, Kapoor V, Basu N, et al. Anti-inflammatory studies on Curcuma longa (turmeric). Indian J Med Res. 1971; 59:1289–1295.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download